Solubility of Fmoc-l-valine in Fourteen Monosolvents: Characterization, Determination, Analysis, and Model Correlation
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
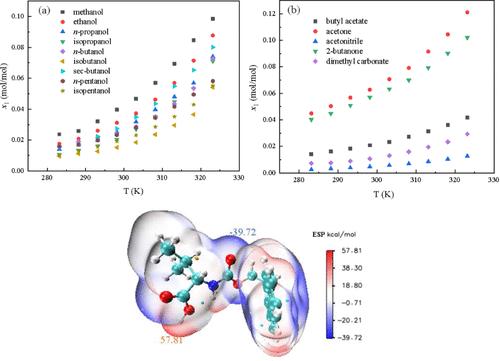
The mole fraction solubility of Fmoc-l-valine in 14 pure solvents (methanol, ethanol n-propanol, isopropanol, n-butanol, isobutanol, sec-butanol, n-pentanol, isopentanol, butyl acetate, acetone, acetonitrile, 2-butanone, dimethyl carbonate) was determined by the static gravimetric method at a temperature range of 283.15 to 323.15 K under a pressure of 101.2 kPa. Among the 14 monosolvents, solubility increased with the increase of absolute temperature. The order of solubility is as follows: acetone (0.06274 mol/mol) > 2-butanone (0.05704 mol/mol) > methanol (0.03966 mol/mol) > ethanol (0.03124 mol/mol) > sec-butanol (0.02749 mol/mol) > n-propanol (0.02535 mol/mol) > n-butanol (0.02445 mol/mol) > n-pentanol (0.02335 mol/mol) > butyl acetate (0.02088 mol/mol) > isopropanol (0.02038 mol/mol) > isopentanol (0.01916 mol/mol) > isobutanol (0.01525 mol/mol) > dimethyl carbonate (0.01078 mol/mol) > acetonitrile (0.004770 mol/mol). The equilibrium solid phase of Fmoc-l-valine in the solvent systems was characterized by powder X-ray diffraction analysis. The solubility data were fitted using the modified Apelblat model, NRTL model, UNIQUAC model, and Margules model; at the same time, the model parameters and data deviation values were calculated. The results showed that the modified Apelblat model had better correlation results. Interaction energy, interaction region indicator (IRI), and molecular electrostatic potential surface (MEPS) were used to determine the internal interactions within Fmoc-l-valine solutions. The Hansen solubility parameters (HSPs) was utilized to assess the solvents’ capability and to elucidate its ability to dissolve Fmoc-l-valine. Furthermore, the mixing thermodynamic characteristics of Fmoc-l-valine in selected solvents were calculated using the NRTL model, which revealed that the mixing process was spontaneous and entropy-driven. The solubility data can be used for the preparation and optimization of the Fmoc-l-valine crystallization processes.
Fmoc-l-valine 在十四种单溶剂中的溶解度:特性、测定、分析和模型相关性
在温度为 283.15 至 323.15 K、压力为 101.2 kPa 的条件下,采用静态重量法测定了 Fmoc-l-valine 在 14 种纯溶剂(甲醇、乙醇、正丙醇、异丙醇、正丁醇、异丁醇、仲丁醇、正戊醇、异戊醇、乙酸丁酯、丙酮、乙腈、2-丁酮、碳酸二甲酯)中的摩尔分数溶解度。在 14 种单溶剂中,溶解度随着绝对温度的升高而增加。溶解度的顺序如下:丙酮(0.06274 摩尔/摩尔);2-丁酮(0.05704 摩尔/摩尔);甲醇(0.03966 摩尔/摩尔);乙醇(0.03124 摩尔/摩尔);仲丁醇(0.02749 摩尔/摩尔);正丙醇(0.02535 摩尔/摩尔);正丁醇(0.02445 mol/mol) > 正戊醇 (0.02335 mol/mol) > 乙酸丁酯 (0.02088 mol/mol) > 异丙醇 (0.02038 mol/mol) > 异戊醇 (0.01916 mol/mol) > 异丁醇 (0.01525 mol/mol) > 碳酸二甲酯 (0.01078 mol/mol) > 乙腈 (0.004770 mol/mol)。粉末 X 射线衍射分析表征了 Fmoc-l-valine 在溶剂体系中的平衡固相。利用改进的 Apelblat 模型、NRTL 模型、UNIQUAC 模型和 Margules 模型对溶解度数据进行了拟合,同时计算了模型参数和数据偏差值。结果表明,修正的 Apelblat 模型具有更好的相关性。利用相互作用能、相互作用区域指示器(IRI)和分子静电位面(MEPS)确定了 Fmoc-l-valine 溶液中的内部相互作用。汉森溶解度参数(HSPs)用于评估溶剂的能力,并阐明其溶解 Fmoc-l-valine 的能力。此外,还利用 NRTL 模型计算了 Fmoc-l-valine 在选定溶剂中的混合热力学特性,结果表明混合过程是自发的、熵驱动的。溶解度数据可用于制备和优化 Fmoc-l-valine 结晶过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


