Assessing Iron Complexation by Dissolved Organic Matter Using Mediated Electrochemical Oxidation
IF 2.9
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
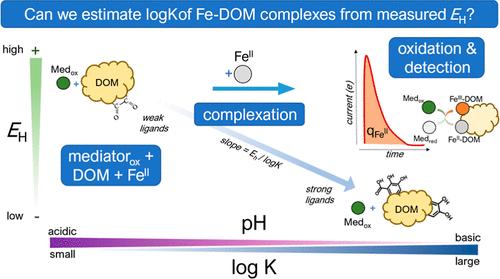
FeII is an abundant reductant in the environment that participates in numerous biogeochemical cycles and pollutant attenuation. FeII in aquatic environments can exist as a complex with dissolved organic matter (DOM), where organic ligands in DOM can modulate iron’s redox potential (EH) and henceforth reactivity as a reductant. Previous studies have assessed the reactivity of FeII-complexes using probe compounds, although these compounds are limited in their ability to profile FeII oxidation across multiple thermodynamic conditions (i.e., both pH and EH) and fail to validate the EH of Fe(II)-complexes via their direct measurement. This study elucidated the redox potentials of FeII-DOM complexes via mediated electrochemical oxidation (MEO) and assessed the extent of FeII oxidation at two different applied EH and pH regimes. Furthermore, we used a Nernstian-based model calibrated with a training set between known iron-ligand thermodynamic stability constants and their respective measured potentials to indirectly determine the stability constants of both FeII and FeIII-DOM complexes as a function of EH and pH. This work highlights the versatility of MEO as an electrochemical technique and is the first to assess stability constants of Fe-complexes with aquatic DOM isolates. We also discuss linkages between speciation modeling and redox reactivity of FeII.
利用介导电化学氧化法评估溶解有机物对铁的络合作用
铁Ⅱ是环境中一种丰富的还原剂,参与许多生物地球化学循环和污染物衰减。水生环境中的 FeII 可以与溶解有机物(DOM)形成络合物,DOM 中的有机配体可以调节铁的氧化还原电位(EH),进而调节铁作为还原剂的反应活性。之前的研究利用探针化合物评估了铁(II)络合物的反应性,但这些化合物在多个热力学条件(即 pH 值和 EH 值)下分析铁(II)氧化的能力有限,而且无法通过直接测量来验证铁(II)络合物的 EH 值。本研究通过介导电化学氧化(MEO)阐明了 FeII-DOM 复合物的氧化还原电位,并评估了在两种不同应用 EH 和 pH 条件下 FeII 的氧化程度。此外,我们还使用了一个基于 Nernstian 的模型,该模型通过已知铁配体热力学稳定常数及其各自测量电位之间的训练集进行校准,从而间接确定了 FeII 和 FeIII-DOM 复合物的稳定常数与 EH 和 pH 值的函数关系。这项工作凸显了 MEO 作为一种电化学技术的多功能性,也是首次评估与水生 DOM 分离物的铁络合物的稳定常数。我们还讨论了 FeII 的物种建模与氧化还原反应性之间的联系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Earth and Space Chemistry
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
5.30
自引率
11.80%
发文量
249
期刊介绍:
The scope of ACS Earth and Space Chemistry includes the application of analytical, experimental and theoretical chemistry to investigate research questions relevant to the Earth and Space. The journal encompasses the highly interdisciplinary nature of research in this area, while emphasizing chemistry and chemical research tools as the unifying theme. The journal publishes broadly in the domains of high- and low-temperature geochemistry, atmospheric chemistry, marine chemistry, planetary chemistry, astrochemistry, and analytical geochemistry. ACS Earth and Space Chemistry publishes Articles, Letters, Reviews, and Features to provide flexible formats to readily communicate all aspects of research in these fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


