Non-Hydroxyl Radical Species Production during Dark Air Oxidation of Alluvial Soils
IF 2.9
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Natural environments subjected to hydrologically driven redox fluctuations are regarded as propitious to contaminant degradation since they favor the cyclic oxygenation of Fe(II) minerals, which produces oxygen reactive species (ROS), such as the hydroxyl radical, OH•. However, the identity of these reactive species may vary as a function of physicochemical conditions and remains a matter of research. Here, using spin-trapping electron paramagnetic resonance (EPR) with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin trap, we show that a non-hydroxyl reactive species is produced in significant amounts upon air-oxidation of alluvial soil suspensions (Seine River Basin, France). Indeed, among the DMPO–OH•, DMPO–CO2•–, and DMPO–alkyl• adducts observed, the latter dramatically increases with the addition of ethanol or, to a lesser extent, tert-butanol, especially with phosphate buffer. This result reveals a dominant non-hydroxyl species, which we interpret as Fe(IV) since it is known to oxidize alcohols to alkyl• radicals and is favored by phosphate ligands. With phosphate buffer and ethanol, the DMPO–alkyl• production correlates with the initial reduced-state iron pool in the samples, as determined using Fe K-edge X-ray absorption spectroscopy (XAS). Fe(II) phyllosilicates, Fe(0) in one soil core and, to a lesser extent, vivianite, are found to be the most significantly oxidized iron phases upon soil oxygenation, and pyrite appears less reactive. Hence, we show that a significant reactive species, differing from OH•, forms upon oxygenation of soil Fe(II) minerals, especially in the presence of soil-sourced phosphate. Our results may therefore call to further directly identify this putative Fe(IV) species and to investigate its ability to degrade organic contaminants in natural environments.
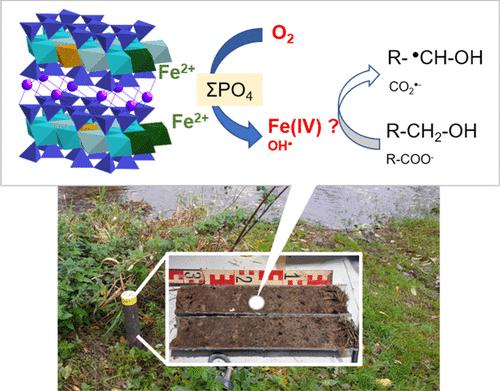
冲积土壤暗空气氧化过程中产生的非羟基自由基物种
受水文驱动的氧化还原波动影响的自然环境被认为有利于污染物降解,因为这些环境有利于铁(II)矿物质的循环氧化,从而产生氧活性物种(ROS),如羟基自由基(OH-)。然而,这些活性物种的特性可能会随着物理化学条件的变化而变化,这仍然是一个研究课题。在这里,我们利用 5,5-二甲基-1-吡咯啉 N-氧化物(DMPO)作为自旋俘获电子顺磁共振(EPR),证明了冲积土壤悬浮物(法国塞纳河流域)在空气氧化过程中会产生大量非羟基反应物。事实上,在观察到的 DMPO-OH-、DMPO-CO2-和 DMPO-烷基-加合物中,后者在加入乙醇或叔丁醇(其次是叔丁醇)后显著增加,尤其是在磷酸盐缓冲液中。这一结果揭示了一种主要的非羟基物种,我们将其解释为 Fe(IV),因为众所周知,Fe(IV)能将醇氧化成烷基自由基,并受到磷酸盐配体的青睐。在磷酸盐缓冲液和乙醇中,DMPO-烷基的生成与样品中的初始还原态铁池相关,这是用铁 K 边 X 射线吸收光谱(XAS)测定的。我们发现,铁(II)层硅酸盐、一个土壤核心中的铁(0)以及少量的维安石是土壤充氧后最显著的氧化铁相,黄铁矿的反应性较低。因此,我们的研究表明,土壤中的铁(II)矿物在充氧后,尤其是在土壤中存在磷酸盐的情况下,会形成一种不同于 OH- 的重要活性物质。因此,我们的研究结果可以进一步直接确定这种推定的铁(IV)物种,并研究其在自然环境中降解有机污染物的能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Earth and Space Chemistry
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
5.30
自引率
11.80%
发文量
249
期刊介绍:
The scope of ACS Earth and Space Chemistry includes the application of analytical, experimental and theoretical chemistry to investigate research questions relevant to the Earth and Space. The journal encompasses the highly interdisciplinary nature of research in this area, while emphasizing chemistry and chemical research tools as the unifying theme. The journal publishes broadly in the domains of high- and low-temperature geochemistry, atmospheric chemistry, marine chemistry, planetary chemistry, astrochemistry, and analytical geochemistry. ACS Earth and Space Chemistry publishes Articles, Letters, Reviews, and Features to provide flexible formats to readily communicate all aspects of research in these fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


