Synthesis and controlled radical polymerization of axially chiral monomers with a binaphthyl skeleton
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
Abstract
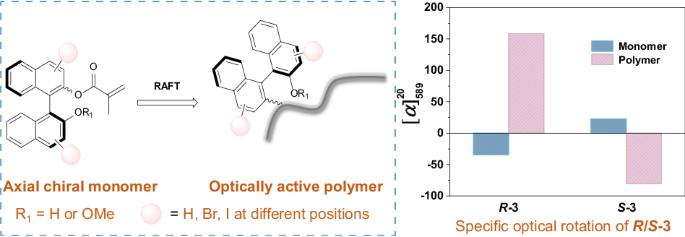
The synthesis of optically active polymers from monomers with chiral central centers is a well-established method, but reports on the synthesis of optically active polymers using axially chiral monomers are limited. In this study, we present the controlled radical polymerization of axially chiral monomers derived from the commercially available 1,1′-bi-2-naphthol (BINOL) structure. A series of optically active polymers based on the axially chiral structures were successfully polymerized using cumyl dithiobenzoate (CDB) as a chain transfer agent (CTA) for the reversible addition-fragmentation chain transfer (RAFT) polymerization. The polymerization process resulted in polymers with high conversions and yield controlled molecular weights. The differences observed in the specific optical rotation and the circular dichroism (CD) signal intensity between the polymers and the monomers confirmed the synthesis of optically active polymers with specific structures. Owing to their unique molecular scaffold, these chiral polymers have promising applications in areas such as chiral separation and catalysis. The graphical abstract shows the controlled radical polymerization of axially chiral monomers bearing a 1,1′-bi-2-naphthol (BINOL) skeleton. The evident increase in magnitude and opposite deflection direction of the specific optical rotation between the synthesized polymer and the monomer confirms the synthesis of the chiral polymers with specific structures.
具有双萘基骨架的轴向手性单体的合成和受控自由基聚合反应
利用具有手性中心的单体合成具有光学活性的聚合物是一种行之有效的方法,但利用轴向手性单体合成具有光学活性的聚合物的报道却很有限。在本研究中,我们介绍了从市售的 1,1′-双-2-萘酚(BINOL)结构中提取的轴向手性单体的受控自由基聚合。使用二硫代苯甲酸积酯(CDB)作为链转移剂(CTA)进行可逆加成-断裂链转移(RAFT)聚合,成功聚合了一系列基于轴向手性结构的光学活性聚合物。聚合过程产生了高转化率和收率可控分子量的聚合物。通过观察聚合物与单体之间特定光学旋转和圆二色性(CD)信号强度的差异,证实合成了具有特定结构的光学活性聚合物。由于其独特的分子支架,这些手性聚合物在手性分离和催化等领域具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


