Tuning Surface Chemistry in 2D Layered BiOI by Facile Liquid-Phase Exfoliation for Enhanced Photoelectrocatalytic Oxygen Evolution
引用次数: 0
Abstract
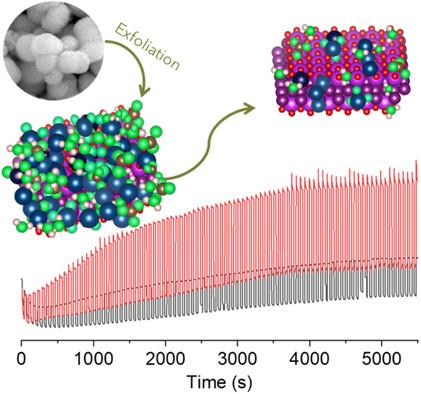
BiOI is a promising photoelectrocatalyst for oxidation reactions. However, the limited photoelectrocatalytic (PEC) activity necessitates the development of new strategies to modify its surface chemistry and thus enhance functional properties. Herein, we present a simple method to increase photocurrent in a BiOI-based photoanode by exfoliating microspheres of the oxyhalide produced through hydrothermal synthesis. Following exfoliation in isopropanol, the resulting layered BiOI-based colloid contains a greater variety of species, including Bi2O2CO3, I3−, IO3−, Bi5+, and hydroxides, compared to the original BiOI. These additional species do not directly enhance the PEC oxygen evolution reaction (OER) performance. Instead, they are consumed or converted during PEC OER, resulting in more active sites on the photoelectrode and reduced resistance, which ultimately improves the water oxidation performance of the exfoliated BiOI. Over long-term chronoamperometry, the exfoliated BiOI demonstrates a photocurrent twice as high as that of the BiOI microspheres. Analysis of the species after PEC OER reveals that the combination of IO3−, Bi5+, and I3− species on the BiOI is beneficial for charge transfer, thus enhancing the intrinsic PEC properties of the BiOI. This study offers new insights into the role of surface chemistry in determining PEC performance, aiding the optimization of 2D materials-based photoelectrocatalysts.
通过便捷的液相剥离技术调节二维层状生物氧化物的表面化学性质,从而增强光电催化氧进化能力
BiOI 是一种用于氧化反应的前景广阔的光电催化剂。然而,由于其光电催化(PEC)活性有限,因此有必要开发新的策略来改变其表面化学性质,从而增强其功能特性。在此,我们提出了一种简单的方法,通过剥离水热合成产生的氧卤化物微球,提高基于 BiOI 的光阳极的光电流。在异丙醇中剥离后,与原始 BiOI 相比,生成的层状 BiOI 基胶体含有更多种类,包括 Bi2O2CO3、I3-、IO3-、Bi5+ 和氢氧化物。这些额外的物种不会直接提高 PEC 氧进化反应(OER)的性能。相反,它们会在 PEC 氧进化反应过程中被消耗或转化,从而在光电极上形成更多的活性位点并降低电阻,最终提高剥离的 BiOI 的水氧化性能。在长期的计时器测量中,剥离的生物氧化物的光电流是生物氧化物微球的两倍。对 PEC OER 后的物种分析表明,BiOI 上 IO3-、Bi5+ 和 I3- 物种的组合有利于电荷转移,从而增强了 BiOI 的内在 PEC 特性。这项研究为了解表面化学在决定 PEC 性能方面的作用提供了新的视角,有助于优化基于二维材料的光电催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


