Scalable 2D Semiconductor-Based van der Waals Heterostructure Interface with Built-in Electric Field for Enhanced Electrochemical Water Splitting
引用次数: 0
Abstract
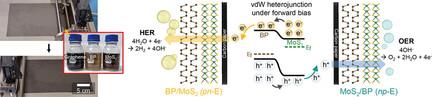
Electrochemical water splitting has received tremendous attention as an eco-friendly approach to produce hydrogen. Noble metals and their oxides are commonly used as electrocatalysts to reduce activation energy barriers for hydrogen and oxygen evolution reactions in high-performance electrodes, but their cost, scarcity, and limited stability hinder widespread adoption of electrochemical water splitting. Further advancements are therefore needed to reduce reliance on noble metals and improve the long-term stability. Herein, solution-processed 2D van der Waals (vdW) p–n heterostructures as an interfacial layer between catalysts and the electrode are introduced to enhance the catalytic performance. These heterostructures are formed by sequentially assembling electrochemically exfoliated black phosphorus and molybdenum disulfide nanosheets into electronic-grade p- and n-type semiconductor thin films, with the scalability extending across tens-of-centimeter scale areas. Benefiting from the charge distribution and built-in electric field developed upon heterojunction formation, the vdW heterostructure interfacial layer increases both the catalytic activity and stability of commercial Pt/C and Ir/C catalysts compared to when these catalysts are directly loaded onto electrodes. Additionally, the vdW heterostructure also serves as a template for synthesizing nanostructured Pt and Ir catalysts through electrodeposition, further enhancing the catalytic performance in terms of mass activity and stability.
具有内置电场的可扩展二维半导体范德华异质结构界面,用于增强电化学水分离功能
电化学水分离作为一种生态友好型制氢方法受到了极大关注。贵金属及其氧化物通常用作电催化剂,以降低高性能电极中氢和氧进化反应的活化能障碍,但其成本、稀缺性和有限的稳定性阻碍了电化学分水技术的广泛应用。因此,需要进一步的进步来减少对贵金属的依赖,并提高其长期稳定性。本文引入溶液加工的二维范德华(vdW)p-n 异质结构作为催化剂与电极之间的界面层,以提高催化性能。这些异质结构是通过将电化学剥离的黑磷和二硫化钼纳米片依次组装成电子级 p 型和 n 型半导体薄膜而形成的,其可扩展性可延伸至数十厘米的区域。得益于异质结形成时产生的电荷分布和内置电场,vdW 异质结构界面层提高了商用 Pt/C 和 Ir/C 催化剂的催化活性和稳定性,而不是直接将这些催化剂装载到电极上。此外,vdW 异质结构还可作为通过电沉积合成纳米结构铂和铱催化剂的模板,进一步提高催化活性和稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


