Peptide and Protein Cysteine Modification Enabled by Hydrosulfuration of Ynamide
IF 12.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Efficient functionalization of peptides and proteins has widespread applications in chemical biology and drug discovery. However, the chemoselective and site-selective modification of proteins remains a daunting task. Herein, a highly efficient chemo-, regio-, and stereoselective hydrosulfuration of ynamide was identified as an efficient method for the precise modification of peptides and proteins by uniquely targeting the thiol group of cysteine (Cys) residues. This novel method could be facilely operated in aqueous buffer and was fully compatible with a wide range of proteins, including small model proteins and large full-length antibodies, without compromising their integrity and functions. Importantly, this reaction provides the Z-isomer of the corresponding conjugates exclusively with superior stability, offering a precise approach to peptide and protein therapeutics. The potential application of this method in peptide and protein chemical biology was further exemplified by Cys-bioconjugation with a variety of ynamide-bearing functional molecules such as small molecule drugs, fluorescent/affinity tags, and PEG polymers. It also proved efficient in redox proteomic analysis through Cys-alkenylation. Overall, this study provides a novel bioorthogonal tool for Cys-specific functionalization, which will find broad applications in the synthesis of peptide/protein conjugates.
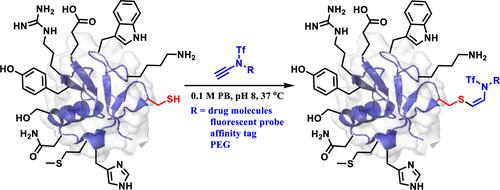
通过亚酰胺的氢硫化作用实现多肽和蛋白质的半胱氨酸修饰
肽和蛋白质的高效功能化在化学生物学和药物发现领域有着广泛的应用。然而,蛋白质的化学选择性和位点选择性修饰仍然是一项艰巨的任务。在此,研究人员发现了一种高效的化学、区域和立体选择性氢化namide 方法,该方法通过独特地靶向半胱氨酸(Cys)残基的硫醇基团,对肽和蛋白质进行精确修饰。这种新方法可在水性缓冲液中轻松操作,并与多种蛋白质完全兼容,包括小型模型蛋白质和大型全长抗体,而不会损害其完整性和功能。重要的是,该反应可提供相应共轭物的 Z-异构体,具有极佳的稳定性,为肽和蛋白质治疗提供了一种精确的方法。这种方法在肽和蛋白质化学生物学中的潜在应用,通过与各种含乙酰胺的功能分子(如小分子药物、荧光/亲和性标签和 PEG 聚合物)进行 Cys 生物共轭得到了进一步体现。通过 Cys-alkenylation 技术,它在氧化还原蛋白质组分析中也被证明是高效的。总之,这项研究为 Cys 特异性功能化提供了一种新的生物正交工具,它将在肽/蛋白质共轭物的合成中得到广泛应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Central Science
Chemical Engineering-General Chemical Engineering
CiteScore
25.50
自引率
0.50%
发文量
194
审稿时长
10 weeks
期刊介绍:
ACS Central Science publishes significant primary reports on research in chemistry and allied fields where chemical approaches are pivotal. As the first fully open-access journal by the American Chemical Society, it covers compelling and important contributions to the broad chemistry and scientific community. "Central science," a term popularized nearly 40 years ago, emphasizes chemistry's central role in connecting physical and life sciences, and fundamental sciences with applied disciplines like medicine and engineering. The journal focuses on exceptional quality articles, addressing advances in fundamental chemistry and interdisciplinary research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


