Antepartum vs postpartum amoxicillin oral challenge in pregnant patients with a reported penicillin allergy: A two-center prospective cohort study
Abstract
Introduction
While 10% of pregnant individuals report a penicillin allergy, there is no established best practice for penicillin allergy delabeling in pregnancy. To better understand options for penicillin delabeling, we aimed to evaluate two penicillin allergy delabeling protocols in pregnancy regarding efficacy, adverse events, and patient satisfaction.
Material and Methods
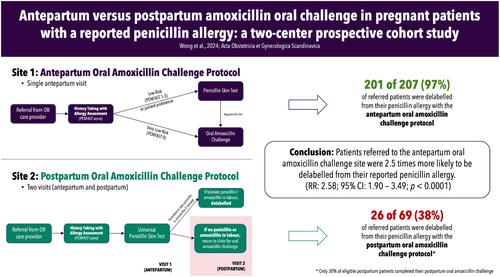
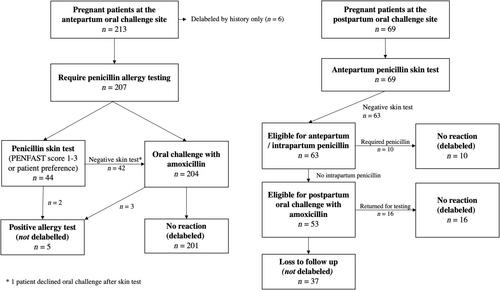
From July 2019 to December 2022, we completed a two-center prospective cohort study, where each site recruited pregnant patients over 24 weeks gestational age with a reported penicillin allergy. One center offered antepartum amoxicillin oral challenges, either directly or after negative skin testing (i.e., antepartum oral challenge site). Our other centers completed a two-step approach with antepartum penicillin skin testing only and deferred oral challenges to the postpartum period (i.e., postpartum oral challenge site). Our primary outcome was the rate of penicillin allergy delabeling, defined as tolerating an antibiotic challenge with penicillin or amoxicillin. Univariate analyses were completed using chi-squared, Fisher's exact, and Wilcoxon rank tests.
Results
During the study period, 276 pregnant patients were assessed, with 207 in the antepartum oral challenge site and 69 in the postpartum oral challenge site. Among the 204 patients who completed antepartum oral challenges, 201 (98%) passed without reactions. Deferring oral challenges to the postpartum period led to a loss of follow-up for 37/53 (70%) of eligible individuals. Overall, 97% (201/207) of patients at the antepartum oral challenge site were delabeled from their penicillin allergy—compared to 38% (26/69) of patients referred to the postpartum oral challenge site (p < 0.0001). Three antepartum oral challenge reactions were noted, including two mild cutaneous reactions and a case of transient abdominal discomfort.
Conclusions
Antepartum amoxicillin oral challenge is a more effective method to delabel pregnant patients from their penicillin allergy. Deferral of oral challenges to the postpartum period introduces a significant barrier for penicillin allergy delabeling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


