Accelerating Kinetics of Alkaline Hydrogen Oxidation Reaction on Ru through Engineering Oxophilicity
IF 8.7
1区 化学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Benefiting from its hydrogen binding energy being similar to that of platinum (Pt), ruthenium (Ru) is expected to replace Pt in the alkaline hydrogen oxidation reaction (HOR). Unfortunately, the adsorbed hydroxyl groups tend to cover Ru surfaces, making Ru unable to adsorb hydrogen, resulting in sluggish HOR kinetics. In this work, we demonstrate that alkaline HOR kinetics on Ru can be accelerated by combining it with highly oxophilic MoO2. Resulting from the stronger oxophilicity of MoO2, hydroxyl groups preferentially adsorb on MoO2, allowing Ru sites to adsorb hydrogen, thus facilitating HOR kinetics. Guided by the theoretical calculations, we synthesize a Ru/MoO2 catalyst for HOR, achieving a mass activity of 5.17 A mgPGM–1, which is 13.6 times greater than that of commercial 20% Pt/C. Notably, a peak power density of 1.09 W cm–2 is achieved using this Ru/MoO2 catalyst as the anode in an anion exchange membrane fuel cell (AEMFC).
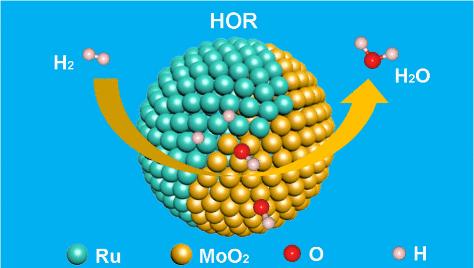
通过亲氧化工程加速 Ru 上碱性氢氧化反应的动力学过程
钌(Ru)的氢结合能与铂(Pt)相似,因此有望在碱性氢氧化反应(HOR)中取代铂。遗憾的是,吸附的羟基往往会覆盖 Ru 表面,使 Ru 无法吸附氢气,从而导致氢氧化反应动力学缓慢。在这项工作中,我们证明了通过将 Ru 与高亲氧化性的 MoO2 结合,可以加速 Ru 上的碱性 HOR 动力学。由于 MoO2 具有更强的亲氧化性,羟基会优先吸附在 MoO2 上,使 Ru 位点能够吸附氢,从而促进 HOR 动力学。在理论计算的指导下,我们合成了用于 HOR 的 Ru/MoO2 催化剂,其质量活性达到 5.17 A mgPGM-1,是商用 20% Pt/C 催化剂的 13.6 倍。值得注意的是,使用这种 Ru/MoO2 催化剂作为阴离子交换膜燃料电池 (AEMFC) 的阳极,可以达到 1.09 W cm-2 的峰值功率密度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Materials Letters
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
14.60
自引率
3.50%
发文量
261
期刊介绍:
ACS Materials Letters is a journal that publishes high-quality and urgent papers at the forefront of fundamental and applied research in the field of materials science. It aims to bridge the gap between materials and other disciplines such as chemistry, engineering, and biology. The journal encourages multidisciplinary and innovative research that addresses global challenges. Papers submitted to ACS Materials Letters should clearly demonstrate the need for rapid disclosure of key results. The journal is interested in various areas including the design, synthesis, characterization, and evaluation of emerging materials, understanding the relationships between structure, property, and performance, as well as developing materials for applications in energy, environment, biomedical, electronics, and catalysis. The journal has a 2-year impact factor of 11.4 and is dedicated to publishing transformative materials research with fast processing times. The editors and staff of ACS Materials Letters actively participate in major scientific conferences and engage closely with readers and authors. The journal also maintains an active presence on social media to provide authors with greater visibility.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


