Enhanced Selectivity in PMS Activation via Non-Metal Doping for Efficient 1O2 Generation in Emerging Organic Pollutants Degradation
IF 7.4
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
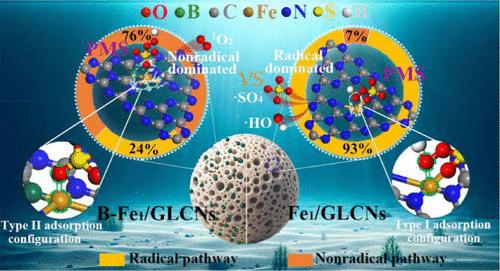
The activation of peroxymonosulfate (PMS) to generate singlet oxygen (1O2) for the removal of emerging organic pollutants (EOPs) from complex aqueous environments has garnered widespread attention. However, the low efficiency and selectivity of current PMS activation for 1O2 generation result in suboptimal EOP degradation. To enhance the selectivity of PMS activation and promote the non-radical pathway, non-metal heteroatoms with varying electronegativities were introduced to disrupt the symmetrical coordination structure of Fe active sites in Fe single-atom catalysts. The results showed that, in the B-Fe1/GLCNs/PMS system, the pseudo-first-order kinetic rate for bisphenol A (BPA) degradation reached 4.435 min–1, which is 7.4 times higher than that of the unmodified control group. Experimental and theoretical calculations demonstrated that the doping of non-metal heteroatoms altered the electron density and distribution at the Fe active sites, thereby modulating the adsorption configuration of HSO5– and increasing the selectivity for PMS activation to generate 1O2. Additionally, the degradation of EOPs by 1O2 produced intermediate products with lower biological toxicity, and 1O2 demonstrated strong anti-interference capability. The change in HSO5– morphology improved the rate of 1O2 generation. This study provides deep insights into designing high-performance PMS activation catalysts via non-metal doping to regulate the electronic structure of active sites for a selective non-radical pathway.
通过非金属掺杂提高 PMS 活化的选择性,从而在新兴有机污染物降解过程中高效生成 1O2
活化过一硫酸盐(PMS)以产生单线态氧(1O2),从而去除复杂水环境中的新有机污染物(EOPs)的方法受到了广泛关注。然而,目前 PMS 激活生成 1O2 的效率和选择性较低,导致 EOP 降解效果不理想。为了提高 PMS 活化的选择性并促进非自由基途径,研究人员引入了不同电负性的非金属杂原子,以破坏铁单原子催化剂中铁活性位点的对称配位结构。结果表明,在 B-Fe1/GLCNs/PMS 体系中,双酚 A(BPA)降解的伪一阶动力学速率达到 4.435 min-1,是未改性对照组的 7.4 倍。实验和理论计算表明,非金属杂原子的掺杂改变了铁活性位点的电子密度和分布,从而改变了 HSO5- 的吸附构型,提高了 PMS 活化生成 1O2 的选择性。此外,1O2 对 EOP 的降解产生了生物毒性较低的中间产物,并且 1O2 表现出很强的抗干扰能力。HSO5- 形态的改变提高了 1O2 的生成速率。这项研究为通过非金属掺杂来调节活性位点的电子结构以实现选择性非辐射途径,从而设计高性能 PMS 活化催化剂提供了深刻的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS ES&T engineering
ENGINEERING, ENVIRONMENTAL-
CiteScore
8.50
自引率
0.00%
发文量
0
期刊介绍:
ACS ES&T Engineering publishes impactful research and review articles across all realms of environmental technology and engineering, employing a rigorous peer-review process. As a specialized journal, it aims to provide an international platform for research and innovation, inviting contributions on materials technologies, processes, data analytics, and engineering systems that can effectively manage, protect, and remediate air, water, and soil quality, as well as treat wastes and recover resources.
The journal encourages research that supports informed decision-making within complex engineered systems and is grounded in mechanistic science and analytics, describing intricate environmental engineering systems. It considers papers presenting novel advancements, spanning from laboratory discovery to field-based application. However, case or demonstration studies lacking significant scientific advancements and technological innovations are not within its scope.
Contributions containing experimental and/or theoretical methods, rooted in engineering principles and integrated with knowledge from other disciplines, are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


