Synthesis and biological evaluation of natural Lachnophyllum methyl ester, Lachnophyllum lactone and their synthetic analogs†
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
(2Z)-Lachnophyllum methyl ester and (4Z)-Lachnophyllum lactone were recently identified as major components in essential oils and extracts of Conyza bonariensis from Togo. Extended biological evaluation of these acetylenic compounds was however hampered by the reduced amounts isolated. A synthetic route was designed providing access to larger quantities of these two natural products as well as to original non-natural analogs with the prospect of exploring for the first time the structure–activity relationships in this series. Using LC/MS analysis, synthetic samples allowed confirming the presence of the two previously isolated natural products in plant extracts obtained by the accelerated solvent extraction technique. The nematocidal activity of the synthesized compounds confirmed the potency of the natural products, which remain the most active among all analogs tested. The synthesized compounds were also assessed against Leishmania infantum axenic amastigotes and the Mycobacterium tuberculosis H37Rv pathogenic strain. (2Z)-Lachnophyllum methyl ester, (4Z)-Lachnophyllum lactone and lactone analogs exhibited the strongest antileishmanial potency. As expected, a longer alkyl chain was necessary to observe significant antimycobacterial activity. The lactone analog bearing a C10 lipophilic appendage displayed the highest antimycobacterial potency. The notable activities of lactones, naturally occurring or analogs, either nematicidal, antileishmanial or antimycobacterial, were compared to their cytotoxicity for mammalian cells and revealed moderate selectivity index values. In this regard, the innocuous (2Z)-Lachnophyllum methyl ester and its analogs open up more promising perspectives for the discovery of bioactive agents to protect both agricultural crops and human health.
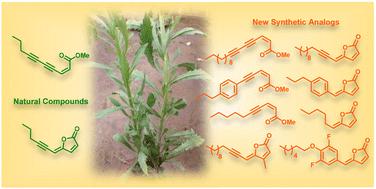
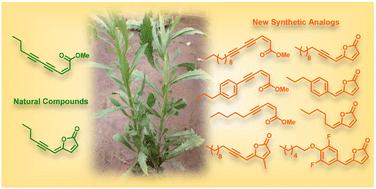
天然叶黄素甲酯、叶黄素内酯及其合成类似物的合成和生物学评价
最近发现,(2Z)-落叶松甲酯和(4Z)-落叶松内酯是多哥 Conyza bonariensis 的精油和提取物中的主要成分。然而,由于分离出的乙炔化合物数量较少,对这些乙炔化合物的生物学评估受到了阻碍。我们设计了一条合成路线,可以获得更大量的这两种天然产物以及原始的非天然类似物,有望首次探索该系列的结构-活性关系。利用 LC/MS 分析法,合成样品证实了通过加速溶剂萃取技术获得的植物提取物中存在这两种先前分离的天然产品。合成化合物的杀线虫活性证实了天然产物的效力,在所有测试的类似物中,天然产物的活性最高。此外,还评估了合成化合物对幼年利什曼病轴状无丝分裂体和结核分枝杆菌 H37Rv 致病菌株的活性。(2Z)-Lachnophyllum 甲酯、(4Z)-Lachnophyllum 内酯和内酯类似物表现出最强的抗利什曼病效力。正如预期的那样,要观察到显著的抗霉菌活性,必须有较长的烷基链。带有 C10 亲脂附属物的内酯类似物显示出最高的抗霉菌效力。将天然内酯或类似物的显著杀线虫、抗利什曼病或抗霉菌活性与它们对哺乳动物细胞的细胞毒性进行比较,结果显示出中等的选择性指数值。因此,无害的 (2Z)-Lachnophyllum 甲酯及其类似物为发现保护农作物和人类健康的生物活性剂开辟了更广阔的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


