Synthesis of the bicyclic butenolide core of pallamolide A: a biomimetic approach†
IF 4.6
Q2 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
Pallamolide A is a 7,8-seco-labdane terpenoid possessing a unique bicyclo[2.2.2]octane core and a spiro-butenolide moiety. A biomimetic synthesis of the bicyclic butenolide core over 10 steps is reported, featuring an unexpected autoxidation ring opening, and a vinylogous Mukaiyama aldol reaction which was spontaneously followed by an unusual intramolecular vinylogous aldol reaction to assemble the spiro-butenolide moiety and bicyclic core of pallamolide A.
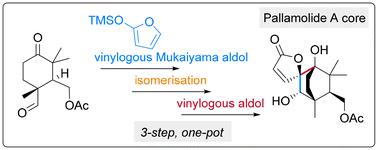
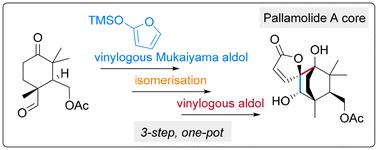
帕拉莫内酯 A 的双环丁烯内酯核心的合成:一种生物模拟方法
帕拉莫内酯 A 是一种 7,8-seco-labdane萜类化合物,具有独特的双环[2.2.2]辛烷核心和一个螺丁烯内酯分子。本研究报告通过 10 个步骤对双环丁烯内酯核心进行了生物模拟合成,其特点包括意想不到的自氧化开环和乙烯基 Mukaiyama 醛醇反应,该反应之后自发地发生了不寻常的分子内乙烯基醛醇反应,从而组装了帕拉莫内酯 A 的螺丁烯内酯分子和双环核心。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


