Photochemical Origins of Iron Flocculation in Acid Mine Drainage
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Acid mine drainage (AMD) raises a global environmental concern impacting the iron cycle. Although the formation of Fe(III) minerals in AMD-impacted waters has previously been reported to be regulated by biological processes, the role of abiotic processes remains largely unknown. This study first reported that a photochemical reaction coupled with O2 significantly accelerated the formation of Fe(III) flocculates (i.e., schwertmannite) in the AMD, as evidenced by the comparison of samples from contaminated sites across different natural conditions at latitudes 24–29° N. Combined with experimental and modeling results, it is further discovered that the intramolecular oxidation of photogenerated Fe(II) with a five-coordinative pyramidal configuration (i.e., [(H2O)5Fe]2+) by O2 was the key in enhancing the photooxidation of Fe(II) in the simulated AMD. The in situ attenuated total reflectance-Fourier transform infrared spectrometry (ATR-FTIR), UV–vis spectroscopy, solvent substitution, and quantum yield analyses indicated that, acting as a precursor for flocculation, [(H2O)5Fe]2+ likely originated from both the dissolved and colloidal forms of Fe(III) through homogeneous and surface ligand-to-metal charge transfers. Density functional theory calculations and X-ray absorption spectroscopy results further suggested that the specific oxidation pathways of Fe(II) produced the highly reactive iron species and triggered the hydrolysis and formation of transient dihydroxo dimers. The proposed new pathways of Fe cycle are crucial in controlling the mobility of heavy metal anions in acidic waters and enhance the understanding of complicated iron biochemistry that is related to the fate of contaminants and nutrients.
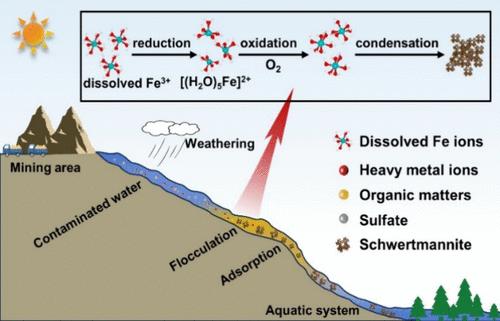
酸性矿井排水中铁絮凝的光化学起源
酸性矿井排水(AMD)是一个影响铁循环的全球性环境问题。尽管之前有报告称,受 AMD 影响的水体中铁(III)矿物的形成受生物过程的调节,但非生物过程的作用在很大程度上仍不为人所知。本研究首次报道了光化学反应与 O2 的耦合显著加速了 AMD 水体中铁(III)絮凝物(即 schwertmannite)的形成、结合实验和建模结果,进一步发现 O2 对具有五配位金字塔构型(即 [(H2O)5Fe]2+)的光生成铁(II)的分子内氧化作用是增强模拟 AMD 中铁(II)光氧化作用的关键。原位衰减全反射-傅立叶变换红外光谱法(ATR-FTIR)、紫外-可见光谱法、溶剂置换和量子产率分析表明,作为絮凝的前体,[(H2O)5Fe]2+ 可能来自溶解和胶体形式的 Fe(III),通过均相和表面配体到金属的电荷转移而产生。密度泛函理论计算和 X 射线吸收光谱结果进一步表明,Fe(II) 的特定氧化途径产生了高活性铁物种,并引发了水解和瞬时二羟基二聚体的形成。所提出的铁循环新途径对于控制重金属阴离子在酸性水体中的流动性至关重要,并加深了人们对与污染物和营养物质归宿相关的复杂铁生物化学的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


