Enhanced stability and electrochemical performance of O3-type NaNi1/3Fe1/3Mn1/3O2 cathode material via yttrium doping for advanced sodium-ion batteries
Abstract
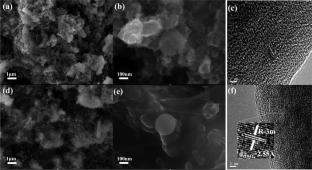
Overcoming rapid capacity decay and structural instability remains a key challenge for the commercialization of O3-type layered cathode materials. This study investigates the effect of yttrium (Y) doping on the performance of NaNi1/3Fe1/3Mn1/3O2 (NNFMO) cathode material for sodium-ion batteries. Compared to the TM-O bonds in the unmodified material, the Y-doped material has stronger Y–O bonds that form a stable structure. Y doping enhances the reversibility of Ni/Fe redox reactions and mitigates the irreversible P3-O'-P3' phase transition. Electrochemical analysis reveals that the Y-doped cathode material (NNFY-10000) exhibits excellent rate performance and remarkable cycle stability. Specifically, NNFY-10000 maintains a discharge capacity of 110.6 mAhg−1 at a 1 C rate and retains 72.26% of its capacity after 200 cycles, outperforming undoped NNFMO. These improvements are attributed to the stable structure formed by strong Y–O bonds, reduced polarization during the cycling process, and enhanced redox reaction reversibility due to Y doping. This study not only elucidates the mechanism by which Y doping improves the electrochemical performance of NNFMO but also provides valuable insights for the development of high-performance sodium-ion battery cathode materials. The strategic introduction of rare earth elements such as Y offers a promising approach to overcoming the inherent limitations of O3-type layered cathode materials, paving the way for their practical application in energy storage systems.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


