Exploring the capacitance of a novel nickel fluoride hydroxide nanomaterial in aqueous solutions
Abstract
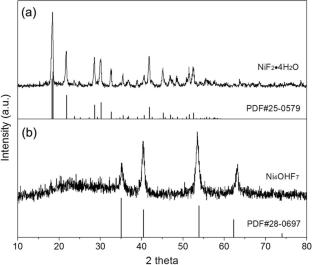
The pursuit of an electrode material that is electrochemically active in aqueous solutions and possesses both high energy density and abundant resources is critical for the development of aqueous hybrid capacitors, which are integral to clean energy storage systems. In this study, we introduce for the first time a novel nickel compound with two anions, displaying a high theoretical energy density—the Ni4OHF7 nanomaterial. We provide a comprehensive analysis of its structure and its electrochemical performance in alkaline aqueous solutions. The Ni4OHF7 nanomaterial is found to be composed of particles approximately 20 nm in size. The electrochemical behavior of Ni4OHF7 in alkaline aqueous solution is mediated by redox reactions occurring between Ni4+ and Ni3+, as well as between Ni3+ and Ni2+. The electrochemical mechanism is primarily diffusion-controlled faradic intercalation process. The study delves into the impact of charge–discharge regulations. Notably, the voltammetric specific capacitance reaches 84.5 F g −1 at a scan rate of 0.03 V s −1, while the discharge specific capacitance is as high as 1128.3 F g −1 at a current density of 1 A g −1. The voltammetric specific capacitance initially increases along with cycling before diminishing and ultimately stabilizes at approximately 30 F g −1 at a scan rate of 0.1 V s −1. The decline in capacitance is attributed to the progressive increase of the charge transfer resistance. The assembled activated carbon (AC)/Ni4OHF7 hybrid capacitor exhibits superior energy and power densities (103.0 Wh kg −1, 7560.7 W kg −1).


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


