Synthesis and Keto-Enol Tautomerism of 1-Phenyl(1-thiophen-2-yl)-4-(diphenylphosphoryl)butane-1,3-diones
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Novel 1-phenyl(1-thiophen-2-yl)-4-(diphenylphosphoryl)butane-1,3-diones were synthesized by the Claisen-type condensation of 1-(diphenylphosphoryl)propan-2-one with ethyl phenyl(thiophene-2-yl)carboxylates. The tautomeric equilibrium of diketone moiety of phosphine oxides was investigated by 1H and 13C{1H} NMR spectroscopy. Two species out of five possible tautomers (diketo and mono-enol) were observed in the solution of 1-phenyl(1-thiophen-2-yl)-4-(diphenylphosphoryl)butane-1,3-diones. The major tautomer was the mono-enol form with content 80–90%. Furthermore, the double bond position at C3 atom was determined by 13C{1H} NMR spectroscopy.
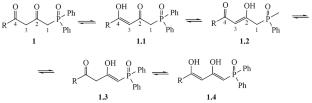
1-苯基(1-噻吩-2-基)-4-(二苯基磷酰)丁烷-1,3-二酮的合成与酮烯醇同分异构体
AbstractNovel 1-苯基(1-噻吩-2-基)-4-(二苯基磷酰)丁烷-1,3-二酮是通过 1-(二苯基磷酰)丙-2-酮与苯基(噻吩-2-基)羧酸乙酯的克莱森式缩合合成的。通过 1H 和 13C{1H} NMR 光谱研究了膦氧化物中二酮分子的同分异构平衡。核磁共振光谱进行了研究。在 1-苯基(1-噻吩-2-基)-4-(二苯基磷酰)丁烷-1,3-二酮的溶液中,观察到五种可能的同分异构体中的两种(二酮和单烯醇)。主要的同分异构体是单烯醇形式,含量为 80-90%。此外,13C{1H}核磁共振光谱测定了 C3 原子的双键位置。核磁共振光谱。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


