Electrochemically promoted selenocyclization for the synthesis of organoselenyl isoxazoles†
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
An electrochemically promoted protocol has been successfully developed for the synthesis of 4-organoselenyl isoxazoles via the oxidative selenocyclization of 2-alkyn-1-one O-methyloximes with diorganyl diselenides as selenation reagents. A wide range of titled compounds were obtained in over 80% yields without an additional catalyst or external oxidant. In comparison with existing methods, this newly developed protocol featured broader substrate scopes, higher selectivity and more atom economy.
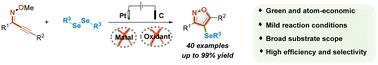
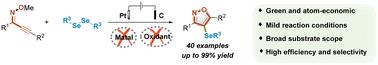
电化学促进硒环化法合成有机硒基异噁唑
以二奥尔加尼二硒化物为硒化试剂,通过 2-炔-1-酮 O-甲基肟的氧化硒环化反应,成功开发出一种电化学促进的 4-有机硒基异噁唑合成方法。在不使用额外催化剂或外部氧化剂的情况下,就能以超过 80% 的产率获得各种标题化合物。与现有方法相比,这种新开发的方法具有底物范围更广、选择性更高、原子更经济的特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


