Acid-catalyzed regioselective remote heteroarylation of alkenes via CC bond migration†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We report herein the acid-catalyzed regioselective remote heteroarylation and reductive alkylation of alkenes. Various alkenes, including mono-, di-, and tri-substituted alkenes and cyclic alkenes, are applicable to this reaction. This method exhibits broad substrate scope, high functional group tolerance and high atomic economy. In addition, both gram-scale synthesis and product transformations demonstrate the potential utility of this reaction. It also provides an efficient and novel pathway to establish the valuable framework of 1,1-diaryl alkanes. Further mechanistic studies suggested that TfOH in situ generated by Cu(OTf)2 and trace H2O catalyzed the alkene migration. Then, heteroarylation was conducted by Friedel–Crafts type alkylation.
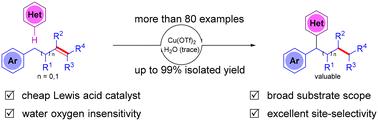
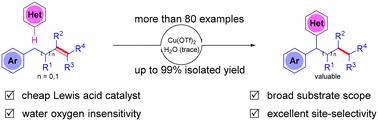
通过 CC 键迁移实现酸催化烯烃的区域选择性远程杂芳基化
我们在此报告了酸催化的烯烃区域选择性远程杂芳基化和还原烷基化反应。包括一、二、三取代烯烃和环烯烃在内的各种烯烃都适用于该反应。这种方法具有底物范围广、官能团耐受性高和原子经济性高等特点。此外,克级规模的合成和产物转化都证明了这一反应的潜在用途。它还为建立有价值的 1,1-二芳基烷烃框架提供了一条高效而新颖的途径。进一步的机理研究表明,由 Cu(OTf)2 和痕量 H2O 原位生成的 TfOH 催化了烯的迁移。然后,通过 Friedel-Crafts 型烷基化反应进行杂芳基化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


