anti-Dihydroxylation of olefins enabled by in situ generated peroxyacetic acid†
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Herein, we report a general and green protocol for the anti-dihydroxylation of unactivated alkenes. Combining H2O2 and acetic acid at 50 °C results in the formation of peroxyacetic acid, which enables the efficient synthesis of a wide range of anti 1,2-diols in moderate to good yields without the need for hazardous solvents or expensive transition metals as catalysts.
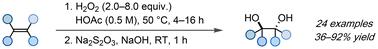
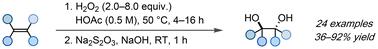
利用原位生成的过氧乙酸实现烯烃的反二羟基化
在此,我们报告了一种用于未活化烯烃反二羟基化的通用绿色方案。将 H2O2 和乙酸在 50 °C的温度下结合会生成过氧乙酸,过氧乙酸能以中等到良好的产率高效合成各种抗 1,2-二醇,而无需使用有害溶剂或昂贵的过渡金属作为催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


