Radical cascade synthesis of γ-amino acids or γ-lactams via carboxyl-mediated intramolecular C–H amination†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The γ C–H amination of carboxylic acid presents a promising and sustainable strategy for synthesizing high-value pharmaceutical chemicals. Radical reaction pathways initiated by aroyloxy radical-involved hydrogen atom transfer (HAT) provide diverse but challenging opportunities for remote C–H functionalization. In this report, the first example of intramolecular γ C–H amination of carboxylic acids using a commercially available oxime auxiliary has been achieved. This innovative approach employs a radical relay chaperone, facilitating selective C–H functionalization via 1,5-HAT/radical cross-coupling and enabling the net incorporation of ammonia at the γ carbon of carboxylic acids. In addition, this protocol enables the recycling of the by-product benzophenone, and both product isolation and by-product recycling are silica gel-free. The reactions offer high chemo- and regio-selectivities, operate under mild reaction conditions, boast a broad substrate scope, exhibit good functional group compatibility, and are easily scalable.
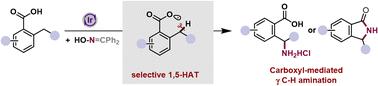
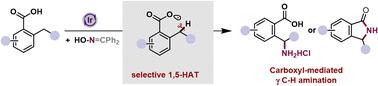
通过羧基介导的分子内 C-H amination,以自由基级联方式合成 γ 氨基酸或 γ 内酰胺
羧酸的 γ C-H amination 是合成高价值医药化学品的一种前景广阔且可持续的策略。由酰氧基自由基参与的氢原子转移(HAT)引发的自由基反应途径为远程 C-H 功能化提供了多种多样但极具挑战性的机会。在本报告中,我们首次利用市售的肟助剂实现了羧酸的分子内 γ C-H 氨化反应。这种创新方法采用自由基中继伴侣,通过 1,5-HAT/ 自由基交叉偶联促进选择性 C-H 功能化,并使氨在羧酸的 γ 碳处实现净结合。此外,该方案还能回收副产物二苯甲酮,而且产品分离和副产物回收都不需要硅胶。这些反应具有很高的化学选择性和区域选择性,反应条件温和,底物范围广,官能团兼容性好,易于扩展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


