Improving both activity and stability for direct conversion of cellulose to ethanol by decorating Pt/WOx with mononuclear NbOx†
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Chemocatalytic conversion of cellulose to ethanol provides an alternative route for biofuel production with a theoretical carbon yield of 100%; however, it faces significant challenges of high catalyst cost and poor catalyst stability. In this work, we report a new strategy to decrease the use of expensive noble metals, by decorating mononuclear NbOx on a low-Pt Pt/WOx catalyst surface. The resulting 0.1Nb/0.5Pt/WOx catalyst gave rise to an ethanol yield of 33.7% together with an ethylene glycol yield of 21.8%, and the noble metal efficiency reached 25.90 gethanol gPt−1 h−1, an increase by a factor of 2–10 compared to those in the literature. Moreover, the catalyst stability was significantly enhanced by the decoration of mononuclear NbOx, allowing for recycling at least 7 times without obvious activity decay. Characterization revealed that Pt was highly dispersed at subnanometer and single atom scales, and modification with mononuclear NbOx facilitated hydrogen spillover and created more oxygen vacancies on the WOx surface upon hydrogen reduction, thus generating a higher density of Brønsted acid sites. This effect not only favored cellulose conversion to ethylene glycol but also promoted the hydrogenolysis of ethylene glycol to ethanol.
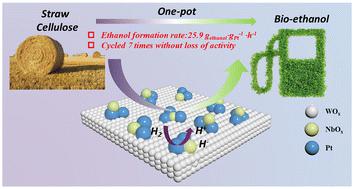
用单核氧化铌装饰铂/氧化物,提高纤维素直接转化为乙醇的活性和稳定性
纤维素到乙醇的化学催化转化为生物燃料的生产提供了另一条途径,其理论碳收率可达 100%;然而,它面临着催化剂成本高和催化剂稳定性差的重大挑战。在这项工作中,我们报告了一种减少使用昂贵贵金属的新策略,即在低铂 Pt/WOx 催化剂表面装饰单核 NbOx。所得到的 0.1Nb/0.5Pt/WOx 催化剂的乙醇产率为 33.7%,乙二醇产率为 21.8%,贵金属效率达到 25.90 gethanol gPt-1 h-1,与文献相比提高了 2-10 倍。此外,通过单核氧化铌的装饰,催化剂的稳定性显著提高,至少可以循环使用 7 次而不会出现明显的活性衰减。表征结果表明,铂在亚纳米和单原子尺度上高度分散,用单核氧化铌修饰可促进氢溢出,并在氢还原时在 WOx 表面产生更多的氧空位,从而产生更高密度的布氏酸位点。这种效应不仅有利于纤维素转化为乙二醇,还能促进乙二醇氢解为乙醇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


