Divergent Assembly of Functionalized Pyrazolo[1,5-a]pyridine Derivatives via [3+2] Cyclization of N-Aminopyridinium Salts with Various Unsaturated Hydrocarbons
IF 5.5
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Two reaction modes for metal-free [3 + 2] cyclization of N-aminopyridinium derivatives with β-alkoxyvinyl trifluoromethylketones have been described through selective C—O or C—O/C—C bond cleavage. This strategy can also be extended to the [3 + 2] cyclization of N-aminopyridinium derivatives with enaminones and bromoalkynes. A broad range of N-aminopyridinium, N-aminoquinolinium, and N-aminoisoquinolinium salts are well tolerated, enabling the divergent synthesis of trifluoroacylated, non-substituted, acylated, and brominated pyrazolo[1,5-a]pyridine derivatives (62 examples).
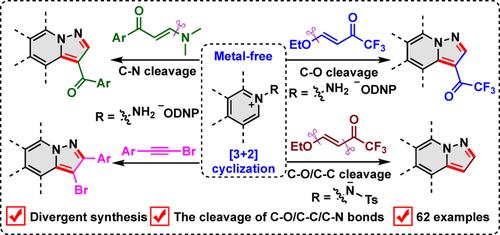
通过 N-氨基吡啶鎓盐与各种不饱和碳氢化合物的 [3+2] 环化作用组装功能化的吡唑并[1,5-a]吡啶衍生物
综合摘要通过选择性 C-O 或 C-O/C-C 键裂解,描述了 N-氨基吡啶鎓衍生物与 β-烷氧基乙烯基三氟甲基酮进行无金属 [3 + 2] 环化的两种反应模式。这种策略还可以扩展到 N-氨基吡啶鎓衍生物与烯胺酮和溴炔的[3 + 2]环化反应。各种 N-氨基吡啶鎓、N-氨基喹啉鎓和 N-氨基异喹啉鎓盐的耐受性都很好,因此可以合成三氟酰化、非取代、酰化和溴化的吡唑并[1,5-a]吡啶衍生物(62 个实例)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Journal of Chemistry
化学-化学综合
CiteScore
8.80
自引率
14.80%
发文量
422
审稿时长
1.7 months
期刊介绍:
The Chinese Journal of Chemistry is an international forum for peer-reviewed original research results in all fields of chemistry. Founded in 1983 under the name Acta Chimica Sinica English Edition and renamed in 1990 as Chinese Journal of Chemistry, the journal publishes a stimulating mixture of Accounts, Full Papers, Notes and Communications in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


