Enhanced Efficiency of Amide-Substituted Quinuclidine-Boranes as Hydridic Hydrogen Atom Transfer Catalysts for Photoinduced Hydroalkylation of Unactivated Olefins†
IF 5.5
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
An amide-substituted quinuclidine-borane has been identified as a more efficient hydridic hydrogen atom transfer (HAT) catalyst for the hydroalkylation of unactivated olefins under visible-light irradiation. 1H NMR titration experiments reveal that the amide moiety of the quinuclidine-borane catalyst forms stronger hydrogen bonds with the carbonyl substrates, thereby improving the reaction yields. Furthermore, it was found that the reaction yields correlate well with the association constant between the quinuclidine-borane catalyst and the carbonyl substrate. A scale-up reaction using a continuous-flow photoreactor has also been demonstrated.
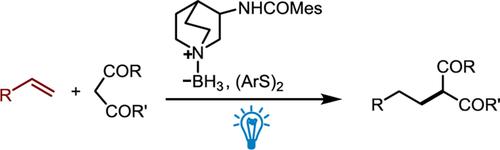
提高酰胺取代的喹啉-硼烷作为氢原子转移催化剂用于未活化烯烃光诱导加氢烷基化的效率† (英文)
综合摘要一种酰胺取代的奎宁环硼烷被鉴定为一种更高效的氢原子转移(HAT)催化剂,可用于在可见光辐照下对未活化烯烃进行氢烷基化反应。1H NMR 滴定实验显示,奎宁环硼烷催化剂的酰胺分子与羰基底物形成了更强的氢键,从而提高了反应产率。此外,研究还发现,反应产率与奎宁环硼烷催化剂和羰基底物之间的结合常数密切相关。此外,还展示了利用连续流光反应器进行的放大反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Journal of Chemistry
化学-化学综合
CiteScore
8.80
自引率
14.80%
发文量
422
审稿时长
1.7 months
期刊介绍:
The Chinese Journal of Chemistry is an international forum for peer-reviewed original research results in all fields of chemistry. Founded in 1983 under the name Acta Chimica Sinica English Edition and renamed in 1990 as Chinese Journal of Chemistry, the journal publishes a stimulating mixture of Accounts, Full Papers, Notes and Communications in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


