Catalytic Asymmetric [4+2] Cyclization of Hydroxyphenyl Indolinone with Azlactone to Construct Spirooxindole δ-Lactone
IF 5.5
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
An efficient asymmetric [4+2] cyclization of hydroxyphenyl indolinone with azlactone for the synthesis of spirooxindole δ-lactone has been developed, which realized the first asymmetric reaction of hydroxyphenyl indolinone. A series of intricate structures with congested vicinal quaternary chiral centers were provided in good yields with excellent enantioselectivities via the in situ generated o-QM from hydroxyphenyl indolinone.
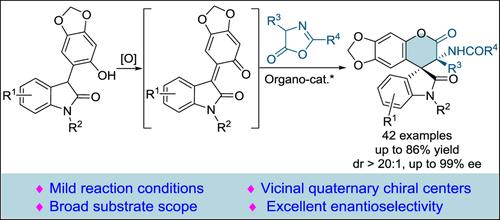
催化羟苯基吲哚啉酮与氮杂环内酯的不对称 [4+2] 环化反应,生成螺吲哚 δ-内酯
综合摘要 研究人员开发了羟基苯基吲哚啉酮与氮内酯的高效不对称[4+2]环化反应,用于合成螺吲哚δ-内酯,实现了羟基苯基吲哚啉酮的首次不对称反应。通过羟基苯基吲哚啉酮原位生成的 o-QM,提供了一系列具有拥挤的副季手性中心的复杂结构,产量高,对映选择性极佳。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Journal of Chemistry
化学-化学综合
CiteScore
8.80
自引率
14.80%
发文量
422
审稿时长
1.7 months
期刊介绍:
The Chinese Journal of Chemistry is an international forum for peer-reviewed original research results in all fields of chemistry. Founded in 1983 under the name Acta Chimica Sinica English Edition and renamed in 1990 as Chinese Journal of Chemistry, the journal publishes a stimulating mixture of Accounts, Full Papers, Notes and Communications in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


