Synthesis of chiral carbocycles via enantioselective β,γ-dehydrogenation
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
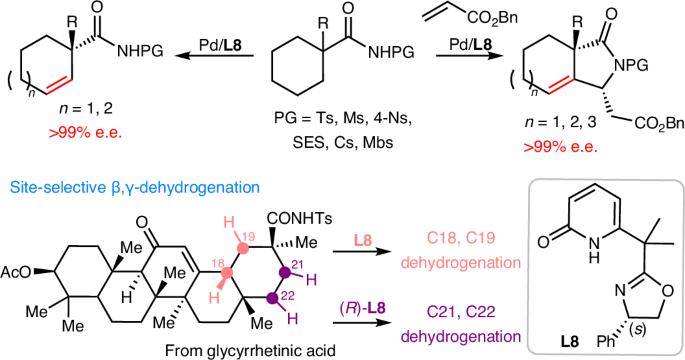
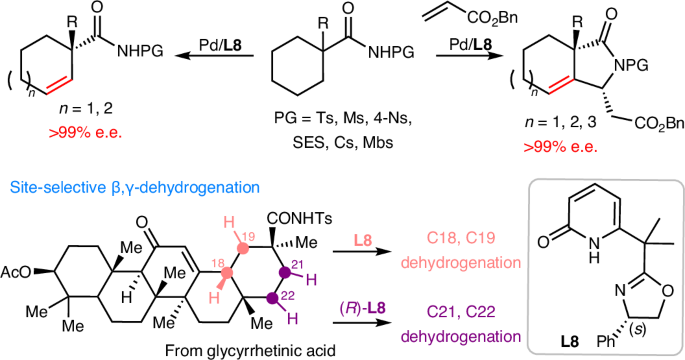
Dehydrogenation of an alkyl group via C–H activation forms a vinyl unit, which can act as a versatile stepping stone for diverse late-stage structural modifications at two adjacent sp3 carbon centres. However, enantioselective dehydrogenation via C–H metalation remains a challenge. Here we describe the realization of palladium-catalysed enantioselective β,γ-dehydrogenation of cycloalkyl amides enabled by chiral oxazoline–pyridone ligands to afford a wide range of highly elaborated carbocycles with exceptional enantioselectivity (>99% e.e.). Notably, the resulting chiral β,γ-unsaturated carbocycles are difficult to access via an inverse electron demand Diels–Alder reaction. Through ligand control, a tandem dehydrogenation and C–H olefination sequence also led to the formation of chiral β-alkylidene-γ-lactams. Remarkably, this catalyst is also compatible with biologically important natural products, including diterpenes and pentacyclic triterpenes, where each enantiomer of our chiral ligand enables site-selective modification at four distinct sites within the E ring. Chiral oxazoline–pyridone ligands enable the enantioselective β,γ-dehydrogenation of cycloalkyl amides, with excellent enantio- and site-selectivity. The palladium-catalysed process provides access to chiral β,γ-unsaturated carbocycles and can also be combined with a C–H olefination step to form chiral γ-lactams.
通过对映选择性 β、γ-脱氢合成手性碳环
烷基通过 C-H 活化脱氢后形成乙烯基单元,可作为在两个相邻 sp3 碳中心进行各种后期结构修饰的多功能垫脚石。然而,通过 C-H 金属化实现对映选择性脱氢仍然是一项挑战。在这里,我们描述了通过手性噁唑啉-吡啶酮配体实现钯催化环烷基酰胺的对映选择性 β,γ-脱氢反应,从而以优异的对映选择性(99% e.e.)得到多种高度精细的碳环。值得注意的是,所得到的手性 β、γ-不饱和碳环很难通过反电子需求 Diels-Alder 反应获得。通过配体控制,串联脱氢和 C-H 烯化顺序也导致了手性 β-亚烷基-γ-内酰胺的形成。值得注意的是,这种催化剂还能与具有重要生物学意义的天然产物(包括二萜和五环三萜)相容,我们的手性配体的每个对映体都能在 E 环的四个不同位点上进行位点选择性修饰。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


