Computational analysis of modular diazotransfer reactions for the development of predictive reactivity models and diazotransfer reagents
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
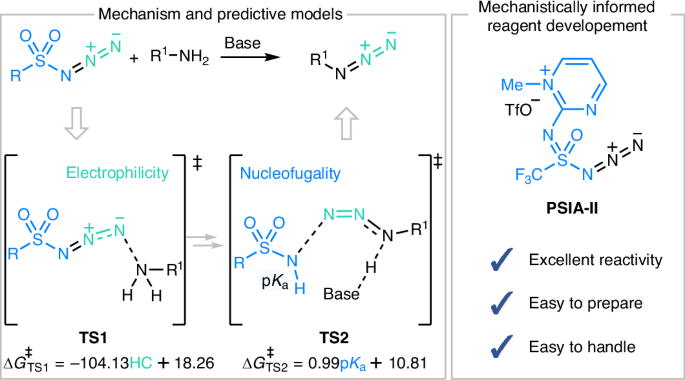
The development of the sulfur(VI)–fluoride exchange (SuFEx) and modular diazotransfer (MoDAT) reactions represent important milestones in the evolution of click chemistry. However, their reactivity profiles, chemoselectivity origins and underlying mechanisms remain unclear. Here we report a computational study of the MoDAT and SuFEx pathways, focusing on the reaction between the diazotransfer reagent fluorosulfuryl azide and primary amines. Our calculations reveal that the MoDAT reaction possesses a small kinetic barrier and a strong driving force, making it kinetically and thermodynamically more favourable than the SuFEx reaction. Through mechanistic scrutiny and structure–activity relationship studies, we have formulated predictive models for the reactivity and selectivity of the MoDAT reaction. Leveraging these insights, an easy-to-prepare and easily handled diazotransfer reagent with excellent reactivity has been developed, which holds broad promise for applications in chemistry and biology. Computational analysis of competing sulfur(VI)–fluoride exchange and modular diazotransfer pathways in the reaction between primary amines and fluorosulfuryl azide reveals that diazotransfer is more kinetically and thermodynamically favoured. Predictive models are formulated by combining mechanistic analysis and structure–activity relationship studies, enabling the development of an easy-to-prepare and highly reactive diazotransfer reagent.

对模块重氮转移反应进行计算分析,以开发预测性反应模型和重氮转移试剂
硫(VI)-氟化物交换(SuFEx)和模块化重氮转移(MoDAT)反应的发展是点击化学发展的重要里程碑。然而,它们的反应性概况、化学选择性起源和内在机制仍不清楚。在此,我们报告了对 MoDAT 和 SuFEx 途径的计算研究,重点是重氮转移试剂氟硫酰叠氮与伯胺之间的反应。我们的计算显示,MoDAT 反应具有较小的动力学障碍和较强的驱动力,使其在动力学和热力学上比 SuFEx 反应更为有利。通过机理分析和结构-活性关系研究,我们为 MoDAT 反应的反应性和选择性建立了预测模型。利用这些洞察力,我们开发出了一种易于制备和处理的重氮转移试剂,它具有极佳的反应活性,在化学和生物学领域有着广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


