Stereospecific C–O sulfation via persulfate-induced 1,4-metallate migration
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Sulfation, a ubiquitous post-translational modification in biomolecules, primarily targets substrates containing OH groups through O-sulfonation (O–SO3). A method for sulfation via the formation of C–O bonds has the potential to access organic sulfates from a broad substrate scope and in a stereoselective manner but remains elusive. Stereospecific C–O bond formation via 1,2-metallate migration in peroxide oxidation has not been deployed to create any other valuable C–O bonds apart from C–OH. Here we describe a fundamentally unique reactivity of persulfate salts for stereospecific C–O sulfation via 1,4-metallate migration. With the aid of readily accessible, stereodefined organic boron compounds derived from native functionalities and a tandem borylation–sulfation approach, our study thus expands to include hydrosulfation of alkenes, C–H sulfation, decarboxylative sulfation, dehalogenative sulfation and deaminative sulfation, which are not otherwise readily accessible. O-sulfonation is a common method for producing organosulfates but is limited to hydroxyl compounds. Here the unique reactivity of persulfates enables stereospecific C–O sulfation via 1,4-metallate migration. This approach provides an unprecedented platform to readily access structurally diverse organosulfates from a wide substrate scope.
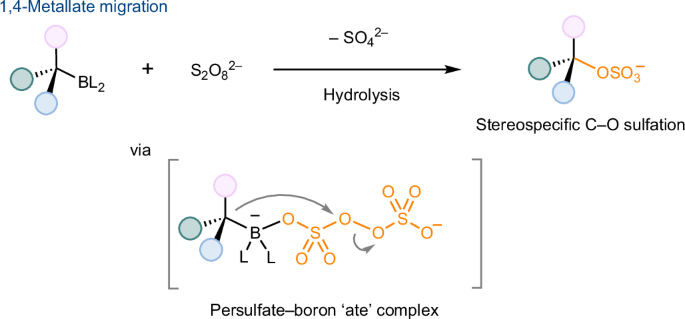

通过过硫酸盐诱导的 1,4-金属铝迁移进行立体特异性 C-O 硫化反应
硫酸化是生物大分子中一种无处不在的翻译后修饰,主要通过 O-磺化(O-SO3)作用于含有 OH 基团的底物。通过形成 C-O 键进行硫酸化的方法有可能以立体选择性的方式从广泛的底物范围中获得有机硫酸盐,但这种方法仍然难以实现。在过氧化物氧化过程中,通过 1,2-Metallate 迁移形成立体特异性 C-O 键的方法,除了 C-OH 之外,还没有用于生成其他有价值的 C-O 键。在这里,我们描述了过硫酸盐通过 1,4-Metallate 迁移进行立体特异性 C-O 硫化的独特反应性。借助从原生官能团中提取的易于获得、立体定义的有机硼化合物以及串联的硼酸化-硫酸化方法,我们的研究由此扩展到包括烯烃的氢化硫酸化、C-H 硫酸化、脱羧硫酸化、脱卤硫酸化和脱氨基硫酸化,而这些反应在其他方面是不容易获得的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


