Structural Landscape and Proton Conduction of Lanthanide 5-(Dihydroxyphosphoryl)isophthalates
IF 3.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
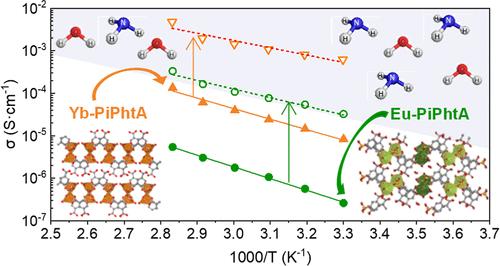
Metal phosphonate-carboxylate compounds represent a promising class of materials for proton conduction applications. This study investigates the structural, thermal, and proton conduction properties of three groups of lanthanide-based compounds derived from 5-(dihydroxyphosphoryl)isophthalic acid (PiPhtA). The crystal structures, solved ab initio from X-ray powder diffraction data, reveal that groups Ln-I, Ln[O3P–C6H3(COO)(COOH)(H2O)2] (Ln = La, Pr), and Ln-II, Ln2{[O3P–C6H3(COO)(COOH)]2(H2O)4}·2H2O (Ln = La, Pr, Eu), exhibit three-dimensional frameworks, while group Ln-III, Ln[O3P–C6H3(COO)(COOH)(H2O)] (Ln = Yb), adopts a layered structure with unbonded carboxylic groups oriented toward the interlayer region. All compounds feature carboxylic groups and coordinating water molecules. Impedance measurements demonstrate that these materials exhibit water-mediated proton conductivity, initially following a vehicle-type proton-transfer mechanism. Upon exposure to ammonia vapors from a 14 or 28% aqueous solution, compounds from groups II and III adsorb ammonia and water, leading to an enhancement in proton conductivity consistent with a Grotthuss-type proton-transfer mechanism. Notably, group II of the studied compounds undergoes the formation of a new expanded phase through the internal reaction of carboxylic groups with ammonia, coexisting with the as-synthesized phase. This postsynthetic modification results in a significant increase in proton conductivity, from approximately ∼5 × 10–6 to ∼10–4 S·cm–1 at 80 °C and 95% relative humidity (RH), attributed to a mixed intrinsic/extrinsic contribution. Remarkably, the NH3(28%)-exposed Yb-III compound achieves an enhancement in proton conductivity, reaching ∼ 5 × 10–3 S·cm–1 at 80 °C and 95% RH, primarily through an extrinsic contribution.
镧系元素 5-(二羟基磷酰)间苯二甲酸盐的结构景观和质子传导
膦酸羧酸金属化合物是一类很有前途的质子传导应用材料。本研究调查了由 5-(二羟基磷酰)间苯二甲酸(PiPhtA)衍生的三组镧系化合物的结构、热和质子传导特性。根据 X 射线粉末衍射数据从头开始求解的晶体结构显示,Ln-I(Ln[O3P-C6H3(COO)(COOH)(H2O)2]组)(Ln = La、Pr)和 Ln-II(Ln2{[O3P-C6H3(COO)(COOH)]2(H2O)4}-2H2O 组)(Ln = La、Pr、Eu)呈现出三元结构、Pr、Eu)呈现出三维框架,而 Ln-III 组 Ln[O3P-C6H3(COO)(COOH)(H2O)](Ln = Yb)则采用层状结构,未键合的羧基朝向层间区域。所有化合物都具有羧基和配位水分子。阻抗测量结果表明,这些材料具有水介导的质子传导性,最初遵循的是载体型质子传输机制。当暴露于 14% 或 28% 水溶液中的氨蒸气时,第 II 组和第 III 组化合物会吸附氨和水,从而导致质子传导性增强,这与 Grotthuss 型质子转移机制是一致的。值得注意的是,所研究化合物的第 II 组通过羧基与氨的内部反应形成了新的扩展相,与合成相共存。在 80 °C 和 95% 相对湿度(RH)条件下,这种合成后修饰导致质子电导率显著增加,从大约 ∼5 × 10-6 增加到 ∼10-4 S-cm-1,这归因于内在和外在的混合作用。值得注意的是,在 80 °C 和 95% 相对湿度条件下,NH3(28%) 暴露的 Yb-III 化合物的质子电导率得到了增强,达到了 ∼ 5 × 10-3 S-cm-1,这主要是通过外在贡献实现的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Crystal Growth & Design
化学-材料科学:综合
CiteScore
6.30
自引率
10.50%
发文量
650
审稿时长
1.9 months
期刊介绍:
The aim of Crystal Growth & Design is to stimulate crossfertilization of knowledge among scientists and engineers working in the fields of crystal growth, crystal engineering, and the industrial application of crystalline materials.
Crystal Growth & Design publishes theoretical and experimental studies of the physical, chemical, and biological phenomena and processes related to the design, growth, and application of crystalline materials. Synergistic approaches originating from different disciplines and technologies and integrating the fields of crystal growth, crystal engineering, intermolecular interactions, and industrial application are encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


