Isothiocyanate Sulfur Atom as an Acceptor Site for Halogen-Bonded Cocrystallization of Werner Ni(II) Coordination Compounds and Perfluorinated Iodobenzenes
IF 3.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We explore the halogen bond acceptor potential of the isothiocyanate sulfur atom in the synthesis of cocrystals involving metal–organic building blocks by using Werner Ni(II) coordination compounds whose pendant isothiocyanate group enables halogen bonding. A series of 14 cocrystals involving octahedral Ni(L)4(NCS)2 coordination compounds (L = pyridine or 4-methylpyridine) has been prepared by both crystallization from solution and liquid-assisted grinding. The effectiveness of this strategy is demonstrated by the assembly of a large family of cocrystals involving five perfluorinated iodobenzenes. For both coordination compounds, we generally obtained one cocrystal with each donor; in one case, we obtained an additional two stoichiomorphs, and in another, we obtained three additional solvates. Single-crystal X-ray diffraction experiments revealed that building units in all cocrystals are connected via S···I halogen bonds involving the donor iodine atom and the isothiocyanate sulfur atom, which is an acceptor of two and, in some cases, even three halogen bonds. Consequently, both coordination compounds act as multitopic acceptors that can form multiple halogen bonds leading to the formation of one-, two-, and three-dimensional halogen-bonded architectures. The relative shortenings of S···I distances are from 7 to 15%, while the S···I–C angles are in the range from 160 to 180°.
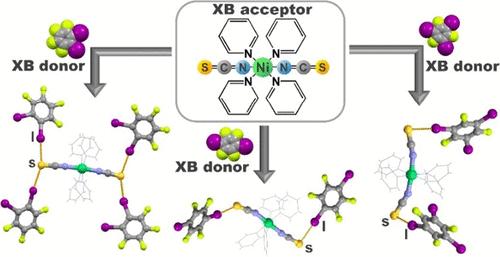
异硫氰酸酯硫原子作为 Werner Ni(II) 配位化合物和全氟碘苯卤键共晶的受体位点
我们利用 Werner Ni(II) 配位化合物(其悬挂的异硫氰酸酯基团可促成卤素键),探索了异硫氰酸酯硫原子在合成涉及金属有机结构单元的共晶体中的卤素键受体潜力。通过从溶液中结晶和液体辅助研磨两种方法,制备了一系列涉及八面体 Ni(L)4(NCS)2 配位化合物(L = 吡啶或 4-甲基吡啶)的 14 颗共晶体。这一策略的有效性体现在我们组装了一大批包含五种全氟碘苯的共晶体。对于这两种配位化合物,我们通常用每个供体获得一种共晶体;在一种情况下,我们获得了另外两种同素异形体,而在另一种情况下,我们获得了另外三种溶解物。单晶 X 射线衍射实验显示,所有共晶体中的结构单元都是通过 S-I 卤素键连接的,其中涉及供体碘原子和异硫氰酸硫原子,而异硫氰酸硫原子是两个卤素键的受体,在某些情况下甚至是三个卤素键的受体。因此,这两种配位化合物都是多位接受体,可以形成多个卤素键,从而形成一维、二维和三维卤素键结构。S-I 间距的相对缩短幅度为 7% 至 15%,而 S-I-C 角的范围为 160° 至 180°。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Crystal Growth & Design
化学-材料科学:综合
CiteScore
6.30
自引率
10.50%
发文量
650
审稿时长
1.9 months
期刊介绍:
The aim of Crystal Growth & Design is to stimulate crossfertilization of knowledge among scientists and engineers working in the fields of crystal growth, crystal engineering, and the industrial application of crystalline materials.
Crystal Growth & Design publishes theoretical and experimental studies of the physical, chemical, and biological phenomena and processes related to the design, growth, and application of crystalline materials. Synergistic approaches originating from different disciplines and technologies and integrating the fields of crystal growth, crystal engineering, intermolecular interactions, and industrial application are encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


