Why Hexanitrobenzene (HNB) Molecules Are Parallelly Stacked along Their Benzene Ring Planes
IF 3.4
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Understanding the molecular structure-crystal packing structure relationship is one of the core objectives of crystal engineering. Hexanitrobenzene (HNB) is a sensitive high-energy compound and presents a surprising molecular stacking structure, i.e., the parallel stacking along their benzene ring planes in crystal seems counterintuitive. HNB molecules each contain six NO2 and a central frame of benzene ring surrounded by the NO2 and feature a same sign-charged molecular edge. Given the electrostatic repulsion among the NO2 groups of adjacent HNB molecules in the crystal, it can hardly make the parallel molecular stacking, as observed experimentally. In the present work, we confirm that the twist of NO2 and the exposure of the molecular frame around the molecular edge are responsible for the aforementioned stacking pattern. Thereon, six negative and six positive electrostatic potential (ESP) regions alternately occur, belonging to the NO2 and exposed molecular frame, respectively. It satisfies a necessary requirement of intralayer molecular arrangement, i.e., the alternate distribution of negative and positive ESP regions on the molecular edge. Meanwhile, the n−π interaction of the NO2·benzene ring governs the interlayer intermolecular interactions. This work is expected to enrich the insight into molecular structure-crystal packing structure relationships.
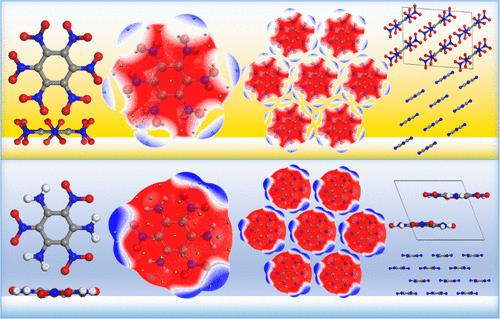
六硝基苯 (HNB) 分子沿苯环平面平行堆积的原因
了解分子结构与晶体堆积结构的关系是晶体工程学的核心目标之一。六硝基苯(HNB)是一种敏感的高能化合物,它呈现出令人惊讶的分子堆积结构,即晶体中沿其苯环平面的平行堆积似乎有悖常理。每个 HNB 分子都包含六个 NO2 和一个由 NO2 包围的苯环中心框架,并具有相同符号电荷的分子边缘。鉴于晶体中相邻 HNB 分子的 NO2 基团之间存在静电排斥,因此很难形成实验观察到的平行分子堆积。在本研究中,我们证实了 NO2 的扭曲和分子边缘周围分子框架的暴露是造成上述堆叠模式的原因。其中,六个负静电势(ESP)区域和六个正静电势(ESP)区域交替出现,分别属于 NO2 和暴露的分子框架。这满足了层内分子排列的一个必要条件,即在分子边缘交替分布负静电势区和正静电势区。同时,NO2-苯环的 n-π 相互作用控制着层间分子间的相互作用。这项工作有望丰富人们对分子结构-晶体堆积结构关系的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Crystal Growth & Design
化学-材料科学:综合
CiteScore
6.30
自引率
10.50%
发文量
650
审稿时长
1.9 months
期刊介绍:
The aim of Crystal Growth & Design is to stimulate crossfertilization of knowledge among scientists and engineers working in the fields of crystal growth, crystal engineering, and the industrial application of crystalline materials.
Crystal Growth & Design publishes theoretical and experimental studies of the physical, chemical, and biological phenomena and processes related to the design, growth, and application of crystalline materials. Synergistic approaches originating from different disciplines and technologies and integrating the fields of crystal growth, crystal engineering, intermolecular interactions, and industrial application are encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


