Differences in Performance among ECPs with Different Metal Centers Based on Highly Stable TzTO
IF 3.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The stability and detonation properties of energetic coordination polymers (ECPs) are usually directly related to the selection of ligands. However, different metals and ligands may form different structures, which will greatly affect the stability and detonation performance of ECPs. Three-dimensional (3D) energetic metal–organic frameworks (EMOFs) usually exhibit enhanced energetic performances and modest stability compared with one-dimensional (1D) and two-dimensional (2D) ECPs. In this work, an energetic internal salt H2TzTO (5-(1H-1,2,4-triazol-4-ium-3-yl)-1H-tetrazol-1-olate) with high stability was selected as the ligand and coordinated with four metal centers, Na(I), K(I), Co(II), and Ni(II), to form three ECPs, named [Na(HTzTO)(H2O)]n·0.5H2O, Co(HTzTO)2(H2O)2, and Ni(HTzTO)2(H2O)2·2H2O, and one EMOF [K(HTzTO)]n, respectively. The four compounds showed excellent thermal stability [decomposition temperature (Td) ≥ 263 °C] and detonation properties [detonation velocity (D) ≥ 8587 m·s–1 and detonation pressure (P) ≥ 33.8 GPa], but their properties are different due to the influence of the metal center and structure. Among them, [K(HTzTO)]n and Co(HTzTO)2(H2O)2 exhibit surprising thermal stability (Td = 344 and 347 °C, respectively) due to the formation of face-to-face π–π stacking in their structures, which greatly enhances the stability of the molecules.
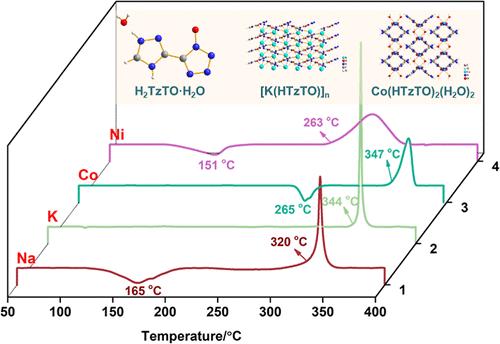
基于高稳定性 TzTO 的不同金属中心 ECP 的性能差异
高能配位聚合物(ECPs)的稳定性和引爆性能通常与配体的选择直接相关。然而,不同的金属和配体可能形成不同的结构,这将极大地影响 ECP 的稳定性和引爆性能。与一维(1D)和二维(2D)ECPs 相比,三维(3D)高能金属有机框架(EMOFs)通常表现出更强的高能性能和适度的稳定性。在这项工作中,我们选择了一种具有高稳定性的高能内盐 H2TzTO(5-(1H-1,2,4-三唑-4-鎓-3-基)-1H-四唑-1-醇)作为配体,并与 Na(I)、K(I)、Co(II) 和 Ni(II) 四种金属中心配位,形成了三种 ECP,分别命名为 [Na(HTzTO)(H2O)]n-0。5H2O、Co(HTzTO)2(H2O)2 和 Ni(HTzTO)2(H2O)2-2H2O,以及一种 EMOF [K(HTzTO)]n。四种化合物均表现出优异的热稳定性[分解温度(Td)≥ 263 ℃]和起爆性能[起爆速度(D)≥ 8587 m-s-1,起爆压力(P)≥ 33.8 GPa],但由于金属中心和结构的影响,它们的性能各不相同。其中,[K(HTzTO)]n 和 Co(HTzTO)2(H2O)2表现出惊人的热稳定性(Td = 344 和 347 ℃),这是因为它们的结构中形成了面对面的 π-π 堆叠,从而大大提高了分子的稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Crystal Growth & Design
化学-材料科学:综合
CiteScore
6.30
自引率
10.50%
发文量
650
审稿时长
1.9 months
期刊介绍:
The aim of Crystal Growth & Design is to stimulate crossfertilization of knowledge among scientists and engineers working in the fields of crystal growth, crystal engineering, and the industrial application of crystalline materials.
Crystal Growth & Design publishes theoretical and experimental studies of the physical, chemical, and biological phenomena and processes related to the design, growth, and application of crystalline materials. Synergistic approaches originating from different disciplines and technologies and integrating the fields of crystal growth, crystal engineering, intermolecular interactions, and industrial application are encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


