Intravitreal long-term sustained ranibizumab delivery using injectable microgel-embedded hydrogel
IF 10.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Retinal vascular disease is the leading cause of visual impairment. Although intravitreal drug injections are the most suitable approach for addressing retinal disorders, existing clinical treatments necessitate repeated administration, imposing a substantial burden on patients with various intraocular complications. This study introduces an injectable and biodegradable hyaluronan microgel (Hm)-embedded gelatin–poly(ethylene glycol)–tyramine hydrogel (HmGh) designed for sustained intravitreal ranibizumab (RBZ) delivery to reduce patient burden and minimize the side effects associated with frequent injections. Hm exhibited a controlled RBZ loading capacity and release profile. HmGh effectively controlled the initial burst release and overall release profile. Cytocompatibility and cellular drug efficacy were also demonstrated. In an animal study, HmGh maintained RBZ concentrations in the vitreous and retina for >120 d. Pharmacokinetic studies showed that the half-life of RBZ-loaded HmGh in the vitreous and retina was 2.55 and 2.05 times longer than that of RBZ-loaded Hm, respectively, and 9.58 and 38.46 times longer than that of RBZ solution, respectively. Importantly, the initial RBZ elimination from HmGh to the aqueous humor was significantly reduced compared to that from the Hm and RBZ solutions. Intraocular degradation and safety were comprehensively evaluated using fundus imaging and histological analyses. In conclusion, this injectable microgel-embedded hydrogel formulation is a promising prolonged drug delivery system for treating various posterior segment eye diseases.
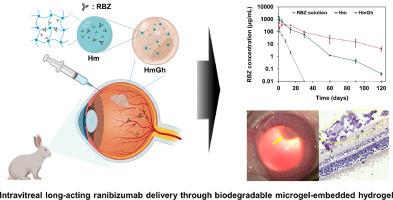
使用可注射微凝胶嵌入水凝胶在玻璃体内长期持续输送雷尼珠单抗
视网膜血管疾病是视力受损的主要原因。虽然玻璃体内注射药物是治疗视网膜病变的最合适方法,但现有的临床治疗方法需要反复用药,给患者带来了很大负担,并引发各种眼内并发症。本研究介绍了一种可注射、可生物降解的透明质酸微凝胶(Hm)--嵌入明胶-聚乙二醇-酪胺水凝胶(HmGh),设计用于持续玻璃体内给药雷尼珠单抗(RBZ),以减轻患者负担并将频繁注射带来的副作用降至最低。Hm 具有可控的 RBZ 装载能力和释放曲线。HmGh 能有效控制初始迸发释放和整体释放曲线。细胞相容性和细胞药效也得到了证实。药代动力学研究表明,负载 RBZ 的 HmGh 在玻璃体和视网膜中的半衰期分别是负载 RBZ 的 Hm 的 2.55 倍和 2.05 倍,是 RBZ 溶液的 9.58 倍和 38.46 倍。重要的是,与 Hm 和 RBZ 溶液相比,RBZ 从 HmGh 进入房水的初始消除量明显减少。眼底成像和组织学分析对眼内降解和安全性进行了全面评估。总之,这种可注射的微凝胶包埋水凝胶配方是一种很有前景的长效给药系统,可用于治疗各种眼后节疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Asian Journal of Pharmaceutical Sciences
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
18.30
自引率
2.90%
发文量
11
审稿时长
14 days
期刊介绍:
The Asian Journal of Pharmaceutical Sciences (AJPS) serves as the official journal of the Asian Federation for Pharmaceutical Sciences (AFPS). Recognized by the Science Citation Index Expanded (SCIE), AJPS offers a platform for the reporting of advancements, production methodologies, technologies, initiatives, and the practical application of scientific knowledge in the field of pharmaceutics. The journal covers a wide range of topics including but not limited to controlled drug release systems, drug targeting, physical pharmacy, pharmacodynamics, pharmacokinetics, pharmacogenomics, biopharmaceutics, drug and prodrug design, pharmaceutical analysis, drug stability, quality control, pharmaceutical engineering, and material sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


