Multi-component ionic diffusion and proton adsorption in charged \({{\upgamma}}\)-alumina structures: Dynamic modeling and experimental study
Abstract
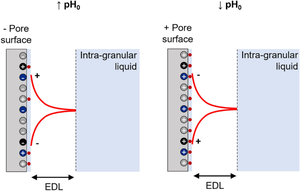
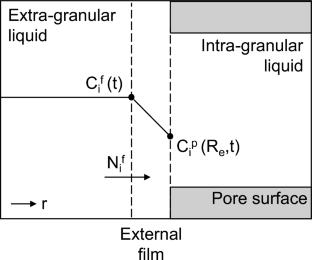
\(\upgamma\)-alumina is highly employed as support for hydrotreatment catalysts prepared by impregnation and as adsorbent in water treatment. These applications consist of contacting \(\upgamma\)-alumina with aqueous solutions, leading to the transport of ions inside the alumina pores and their adsorption on the pore surface. These physicochemical phenomena are governed by the \(\upgamma\)-alumina pore surface and the solution characteristics. Predicting the physicochemical phenomena at the liquid/solid interface is crucial to optimize the design of catalysts and of water treatment adsorption processes. However, this is very challenging using conventional analytical techniques. In this work, the diffusion of protons and their counter-ions inside \(\upgamma\)-alumina pores and the adsorption of protons on the pore surface are modeled at unsteady state during contact with acid solutions at different initial pH levels. Diffusion inside pores is represented using a combination of the zero current method and the Poisson-Boltzmann equation, while the proton adsorption is described by the Langmuir adsorption isotherm. Simulations agree well with the results of proton adsorption experiments in a batch system. The model accurately predicts the distribution of species inside the electrostatic double layer at the liquid/solid interface. It also computes the surface charge and the maximal adsorption capacities of different types of hydroxyl sites present on the alumina pore surface; both are very difficult to determine experimentally. This model can serve as a guide for the comprehension of the liquid/solid interface inside \(\upgamma\)-alumina structures and their interaction with aqueous solutions during the initial stages of impregnation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


