Vanadium Carbide Quantum Dots Exert Efficient Anti-Inflammatory Effects in Lipopolysaccharide-Induced BV2 Microglia and Mice
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
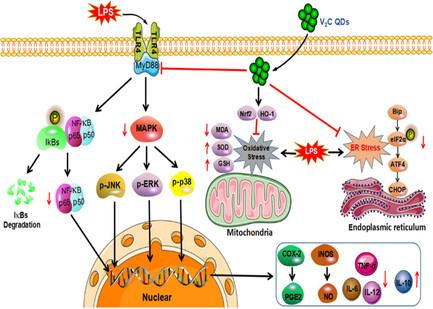
The regulation of glial cell activation is a critical step for the treatment or prevention of neuroinflammation-based brain diseases. However, the development of therapeutic drugs that pass the blood–brain barrier (BBB) and inhibit the glia cell activation remains a significant challenge. Herein, an ultrasmall 2D vanadium carbide quantum dots (V2C QDs) that are capable of crossing the BBB are prepared, and the admirable anti-neuroinflammatory effects are presented. The prepared 2D V2C QDs with an average size of 2.54 nm show good hydrophilicity, physiological stability, and effective BBB-crossing ability. The biological effect of V2C QDs on inflammatory reactions demonstrates fascinating results in preventing the impairment of learning and memory in BALB/c mice stimulated by lipopolysaccharide. Investigation of molecular mechanism reveals that V2C QDs not only inhibit the toll-like receptor 4/myeloid differentiation factor 88-mediated nuclear factor kappa B and mitogen-activated protein kinase pathways, but also prevent eukaryotic translation initiation factor 2α/activating transcription factor 4/C/EBP homologous protein-signaling pathway and reduce oxidative stress via activating the NF-E2-related factor-2/heme oxygenase-1-signaling pathway, leading to greatly inhibited activation of microglia and astrocytes and weakened production of inflammatory cytokines. In summary, V2C QDs exert potent anti-inflammatory effects through multiple pathways, thus offer great potential for the treatment of neurodegenerative diseases.
碳化钒量子点在脂多糖诱导的 BV2 小胶质细胞和小鼠体内发挥高效抗炎作用
调节神经胶质细胞的活化是治疗或预防基于神经炎症的脑部疾病的关键步骤。然而,开发能够通过血脑屏障(BBB)并抑制胶质细胞活化的治疗药物仍然是一项重大挑战。本文制备了能够穿过血脑屏障的超小二维碳化钒量子点(V2C QDs),并介绍了其令人赞叹的抗神经炎症作用。制备的二维碳化钒量子点平均尺寸为 2.54 nm,具有良好的亲水性、生理稳定性和有效的 BBB 穿越能力。V2C QDs 对炎症反应的生物效应在防止脂多糖刺激 BALB/c 小鼠学习和记忆障碍方面取得了令人瞩目的成果。分子机制研究表明,V2C QDs 不仅能抑制由收费样受体 4/髓系分化因子 88 介导的核因子卡巴 B 和丝裂原活化蛋白激酶通路,还能阻止真核细胞翻译、还能阻止真核翻译起始因子2α/激活转录因子4/C/EBP同源蛋白信号通路,并通过激活NF-E2相关因子-2/血红素加氧酶-1信号通路减少氧化应激,从而大大抑制小胶质细胞和星形胶质细胞的活化,削弱炎性细胞因子的产生。总之,V2C QDs 可通过多种途径发挥强大的抗炎作用,因此在治疗神经退行性疾病方面具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


