In Situ Formed Composite Polymer Electrolytes Based on Anion-Trapping Boron Moiety and Polyhedral Oligomeric Silsesquioxane for High Performance Lithium Metal Batteries
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
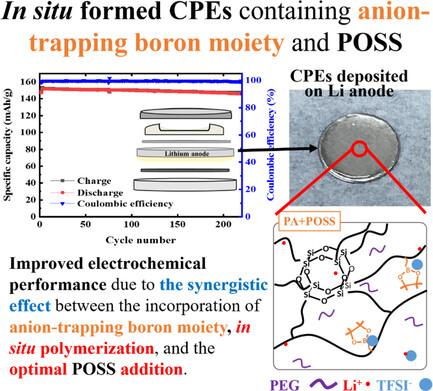
Quasi-solid and composite polymer electrolytes (QSPEs and CPEs) used in lithium-ion battery (LIB) have recently been a novel strategy owing to their high-safety comparing to traditional liquid counterparts. This study reported the preparation of CPEs based on boron moiety, poly(ethylene glycol) (PEG), and octahedral polyhedral oligomeric silsesquioxane (POSS) via in situ thermal polymerization method directly onto the lithium anode to improve the interfacial contact and electrochemical performance. The synergistic effect between the incorporation of anion-trapping boron moiety and in situ polymerization rendered the QSPEs exhibiting higher electrochemical voltage window, ionic conductivity, and transference number as well as better electrochemical performance than the PEG-based counterpart. Due to the Lewis acid effect, anion-trapping boron moiety could promote the dissociation of lithium salts, allowing more lithium ions to be in the free state, thereby enhancing the lithium-ion conductivity. With an optimal addition of POSS, the as-prepared CPEs exhibited lower overpotential during the lithium plating-stripping test and better electrochemical performance than the QSPE counterparts. The optimal POSS addition could facilitate the lithium-ion conduction and establishment of continuous ion pathways, further improving their electrochemical performance. This study pointed a promising approach for developing high performance lithium-ion batteries.
用于高性能锂金属电池的基于阴离子捕获硼分子和多面体低聚硅倍半氧烷的原位形成复合聚合物电解质
与传统的液态电解质相比,锂离子电池(LIB)中使用的准固态和复合聚合物电解质(QSPEs 和 CPEs)具有较高的安全性,因此最近成为一种新的策略。本研究报告了基于硼分子、聚乙二醇(PEG)和八面低聚硅倍半氧烷(POSS)的 CPEs 的制备方法,该方法通过原位热聚合直接作用于锂阳极,以改善界面接触和电化学性能。阴离子捕获硼分子的加入与原位聚合之间的协同效应使 QSPEs 具有更高的电化学电压窗口、离子电导率和转移数,电化学性能也优于 PEG 类产品。由于路易斯酸效应,阴离子捕获硼分子可以促进锂盐解离,使更多的锂离子处于自由状态,从而提高锂离子电导率。在最佳添加 POSS 的情况下,制备的 CPE 在锂电镀剥离测试中表现出较低的过电位,电化学性能也优于 QSPE。最佳的 POSS 添加量可促进锂离子传导并建立连续的离子通道,从而进一步提高其电化学性能。这项研究为开发高性能锂离子电池提供了一种可行的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


