Measurement of Vapor–Liquid Equilibrium Data for Binary Systems CO2 + Ethyl Propionate, Methyl Propionate, Ethyl Acetate, and Ethyl Isobutyrate at 253.15 K
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In order to improve the decarbonization capacity of the absorbent in the syngas purification process in industry, selecting and developing more efficient physical solvents has become a breakthrough direction. Considering that ester solvents have great potential for absorbing CO2, in this work, vapor–liquid equilibrium (VLE) data were measured for the binary system of CO2 + ethyl propionate, methyl propionate, ethyl acetate, and ethyl isobutyrate at 253.15 K. Herein, the operating pressure ranges from 0.3 to 1.7 MPa. The experimental data were correlated by Peng–Robinson (PR) and Soave–Redlich–Kwong (SRK) equations of state with van der Waals (vdW) mixing rules. Among the aforementioned equations, the PR equation of state correlates the experimental data better above 1.2 MPa. Moreover, it can be found that ethyl acetate has the best CO2 absorption performance, followed by methyl propionate, ethyl propionate, and ethyl isobutyrate. In addition, the average absolute relative deviations of the molar fractions of CO2 in the vapor phase and measured pressures were calculated for each system. As a result, the overall deviations were less than 0.02% and 4%, respectively, indicating that the measured experimental values are stable and reliable. This work will not only provide a reference for screening efficient CO2 absorption solvents but also provide the basic thermodynamic data for the syngas purification process.
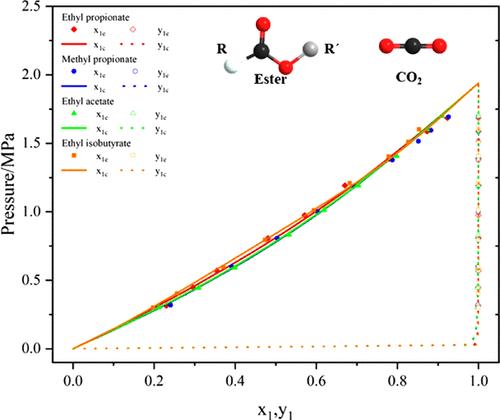
在 253.15 K 温度下测量 CO2 + 丙酸乙酯、丙酸甲酯、乙酸乙酯和异丁酸乙酯二元体系的汽液平衡数据
为了提高工业合成气净化过程中吸收剂的脱碳能力,选择和开发更高效的物理溶剂已成为一个突破方向。考虑到酯类溶剂吸收 CO2 的巨大潜力,本研究在 253.15 K 下测量了 CO2 + 丙酸乙酯、丙酸甲酯、乙酸乙酯和异丁酸乙酯二元体系的汽液平衡(VLE)数据。实验数据通过彭-罗宾逊(PR)和苏韦-雷德里希-邝(SRK)状态方程与范德华(vdW)混合规则进行关联。在上述方程中,PR 状态方程与 1.2 兆帕以上的实验数据相关性较好。此外,可以发现醋酸乙酯的二氧化碳吸收性能最好,其次是丙酸甲酯、丙酸乙酯和异丁酸乙酯。此外,还计算了每个系统气相中二氧化碳摩尔分数与测量压力的平均绝对相对偏差。结果表明,总体偏差分别小于 0.02% 和 4%,表明测量的实验值稳定可靠。这项工作不仅为筛选高效的二氧化碳吸收溶剂提供了参考,还为合成气净化过程提供了基本的热力学数据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


