Adsorption and Diffusion of CH4, N2, and Their Mixture in MIL-101(Cr): A Molecular Simulation Study
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A comprehensive quantitative grasp of methane (CH4), nitrogen (N2), and their mixture’s adsorption and diffusion in MIL-101(Cr) is crucial for wide and important applications, e.g., natural gas upgrading and coal-mine methane capturing. Previous studies often overlook the impact of gas molecular configuration and MIL-101 topology structure on adsorption, lacking quantitative assessment of primary and secondary adsorption sites. Additionally, understanding gas mixture adsorption mechanisms remains a research gap. To bridge this gap and to provide new knowledge, we utilized Monte Carlo and molecular dynamics simulations for computing essential MIL-101 properties, encompassing adsorption isotherms, density profiles, self-diffusion coefficients, radial distribution function (RDF), and CH4/N2 selectivity. Several novel and distinctive findings are revealed by the atomic-level analysis, including (1) the significance of C═C double bond of the benzene ring within MIL-101 for CH4 and N2 adsorption, with Cr and O atoms also exerting notable effects. (2) Density distribution analysis reveals CH4’s preference for large and medium cages, while N2 is evenly distributed along pentagonal and triangular window edges and small tetrahedral cages. (3) Calculations of self-diffusion and diffusion activation energies suggest N2’s higher mobility within MIL-101 compared to CH4. (4) In the binary mixture, the existence of CH4 can decrease the diffusion coefficient of N2. In summary, this investigation provides valuable microscopic insights into the adsorption and diffusion phenomena occurring in MIL-101, thereby contributing to a comprehensive understanding of its potential for applications, e.g., natural gas upgrading and selective capture of coal-mine methane.
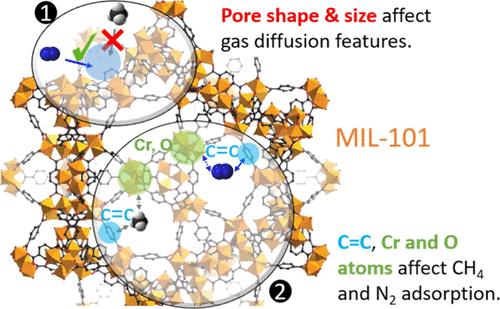
MIL-101(Cr)中 CH4、N2 及其混合物的吸附和扩散:分子模拟研究
全面定量地掌握甲烷(CH4)、氮气(N2)及其混合物在 MIL-101(Cr)中的吸附和扩散情况,对于天然气提纯和煤矿甲烷捕获等广泛而重要的应用至关重要。以往的研究往往忽视气体分子构型和 MIL-101 拓扑结构对吸附的影响,缺乏对主吸附位点和次吸附位点的定量评估。此外,对气体混合物吸附机理的理解仍是一个研究空白。为了弥补这一差距并提供新的知识,我们利用蒙特卡罗和分子动力学模拟计算了 MIL-101 的基本特性,包括吸附等温线、密度曲线、自扩散系数、径向分布函数 (RDF) 和 CH4/N2 选择性。原子级分析揭示了几个新颖独特的发现,包括:(1)MIL-101 中苯环的 C═C 双键对 CH4 和 N2 的吸附具有重要作用,Cr 原子和 O 原子也有显著影响。(2) 密度分布分析表明,CH4 偏爱大型和中型笼,而 N2 则均匀地分布在五边形和三角形窗边以及小型四面体笼中。(3) 自扩散和扩散活化能的计算表明,与 CH4 相比,N2 在 MIL-101 中的流动性更高。(4) 在二元混合物中,CH4 的存在会降低 N2 的扩散系数。总之,这项研究为了解 MIL-101 中发生的吸附和扩散现象提供了宝贵的微观见解,从而有助于全面了解 MIL-101 的应用潜力,例如天然气升级和煤矿甲烷的选择性捕获。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


