Liquid–Liquid Equilibria in Mixtures of 2-Ethylhexanoic Acid, Ethanol, and Water
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Mixtures of 2-ethylhexanoic acid (EHA), ethanol, and water are common constituents of precursor solutions for the spray flame synthesis of nanoparticles. As EHA and water are poorly miscible, the question arises whether phase separation can occur during the process. Since no experimental data on liquid–liquid equilibria (LLE) in mixtures of EHA, ethanol, and water are available in the literature, they were measured in the present work. Binary and ternary LLE were studied at temperatures between 283 and 333 K. A thermodynamic nonrandom two-liquid model (NRTL) was adjusted to the data from this work and to vapor–liquid equilibrium data from the literature. With this model, residue curves in the ternary system were calculated, which indicate that liquid–liquid demixing upon the evaporation of droplets in the spray flame synthesis process is unlikely.
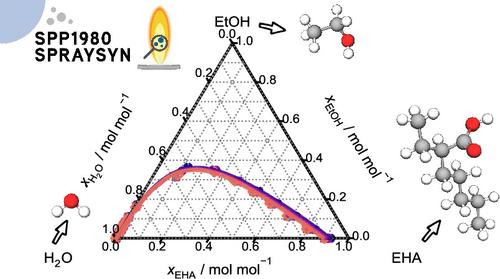
2- 乙基己酸、乙醇和水混合物中的液-液平衡
2- 乙基己酸(EHA)、乙醇和水的混合物是喷雾火焰合成纳米粒子前体溶液的常见成分。由于 EHA 和水的混溶性很差,因此出现了在此过程中是否会发生相分离的问题。由于文献中没有关于 EHA、乙醇和水混合物中液液平衡(LLE)的实验数据,因此本研究对其进行了测量。根据这项研究的数据和文献中的汽液平衡数据,对热力学非随机双液模型(NRTL)进行了调整。利用该模型计算了三元体系中的残留曲线,结果表明在喷雾火焰合成过程中液滴蒸发时不太可能发生液液脱混现象。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


