Adsorption Equilibrium, Kinetics, and Column Breakthrough Data of Acetic Acid, Butyric Acid, and Lactic Acid on the IRN-78 Ion-Exchange Resin at Initial pH (∼3–7) and Temperature (25–55 °C)
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
This work presents systematic aqueous-phase adsorption equilibrium, kinetics, and column breakthrough measurements with three key biointermediates that are common compounds in many bioprocesses. Adsorption equilibrium experiments were carried out with acetic acid, butyric acid, and lactic acid on a commercial ion-exchange resin, Amberlite IRN-78, at wide ranges of acid concentration (8–500 mmol/L), initial pH (∼3–7), and temperature (25–55 °C), simulating the effluent characteristics from different fermenter operations. The kinetics and column breakthrough experiments were conducted at an initial pH of 6 and a concentration of 200 mmol/L. The equilibrium study shows a higher loading at the initial pH < pKa and a lower loading at the initial pH > pKa. Overall removal varies between 16 and 99% depending on the initial pH, temperature, and organic acid concentration and type. The study further indicates monolayer adsorption at the equilibrium pH > 10 and multilayer adsorption at the equilibrium pH < 6. The thermodynamic modeling of adsorption isotherm data was carried out using Langmuir and Freundlich isotherms. IRN-78 presents fast adsorption kinetics as the maximum loading was attained in ≤10 min and nearly the same breakthrough time for all three organic acids involved in this study.
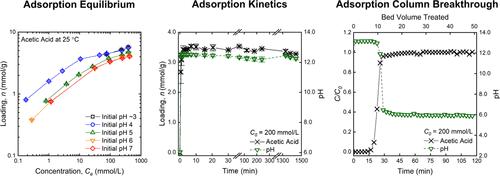
初始 pH 值(∼3-7)和温度(25-55 °C)下 IRN-78 离子交换树脂对乙酸、丁酸和乳酸的吸附平衡、动力学和柱突破数据
本研究对许多生物工艺中常见的三种关键生物中间体进行了系统的水相吸附平衡、动力学和柱突破测量。在商用离子交换树脂 Amberlite IRN-78 上对乙酸、丁酸和乳酸进行了吸附平衡实验,实验条件包括酸浓度(8-500 mmol/L)、初始 pH 值(∼3-7)和温度(25-55 °C),模拟了不同发酵罐操作过程中的流出物特征。在初始 pH 值为 6 和浓度为 200 mmol/L 时进行了动力学和柱突破实验。平衡研究表明,在初始 pH 值为 pKa 时,负载量较高,而在初始 pH 值为 pKa 时,负载量较低。总体去除率在 16% 到 99% 之间,取决于初始 pH 值、温度、有机酸浓度和类型。研究进一步表明,在平衡 pH 值为 10 时为单层吸附,在平衡 pH 值为 6 时为多层吸附。IRN-78 的吸附动力学速度很快,在≤10 分钟内就能达到最大吸附量,而且对本研究涉及的所有三种有机酸的突破时间几乎相同。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


