Isothermal Phase Equilibria of the CH4+CO2 Mixed-Gas Hydrate System for CO2 Capture and Storage in a Reservoir after CH4 Hydrate Exploitation
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
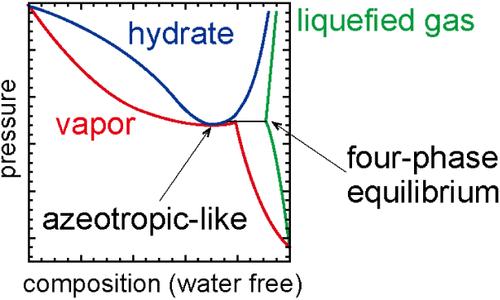
Isothermal phase equilibria of methane (CH4) + carbon dioxide (CO2) mixed-gas hydrate were investigated at 281.98, 284.17, 286.02, and 287.17 K for the carbon dioxide capture and storage (CCS) using gas hydrates in a reservoir after CH4 hydrate exploitation. At temperatures higher than the upper quadruple point Q2 (hydrate + aqueous + CO2-rich liquid + vapor phases) of pure CO2 hydrate, the phase behavior in the CH4 + CO2 mixed-gas hydrate system becomes very complicated due to two characteristic behaviors. One is the four-phase coexistence curve of hydrate + aqueous + CO2-rich liquid + vapor phases in the CH4 + CO2 mixed-gas hydrate system, which originates from Q2 and extends to the critical end point, where it intersects with the vapor–liquid critical locus of the CH4 + CO2 binary system. The other is the negative azeotropic-like retrograde (local pressure minimum) behavior observed at 286.02 and 287.17 K, where the equilibrium pressure of the CH4 + CO2 mixed-gas hydrate is lower than that of either pure CO2 hydrate or pure CH4 hydrate. When CO2 is injected into a CH4-remaining reservoir after CH4 hydrate exploitation, the CO2 composition in the resulting in CH4 + CO2 mixed-gas hydrate phase can increase up to either compositions at the local pressure minimum point (if it exists) or the four-phase equilibrium point at sediment temperatures above Q2 temperature.
开采 CH4 水合物后在储层中捕获和封存 CO2 的 CH4+CO2 混合气体水合物系统的等温相平衡
研究了甲烷 (CH4) + 二氧化碳 (CO2) 混合气体水合物在 281.98、284.17、286.02 和 287.17 K 温度下的等温相平衡,用于开采 CH4 水合物后在储层中使用气体水合物进行二氧化碳捕获和封存 (CCS)。当温度高于纯 CO2 水合物的上四点 Q2(水合物 + 水相 + 富含 CO2 的液相 + 气相)时,CH4 + CO2 混合气体水合物体系中的相行为会变得非常复杂,这是因为它们具有两种特征行为。其一是 CH4 + CO2 混合气体水合物体系中水合物 + 水相 + 富含 CO2 的液相 + 气相的四相共存曲线,该曲线从 Q2 开始一直延伸到临界端点,并与 CH4 + CO2 二元体系的汽液临界点相交。另一种是在 286.02 和 287.17 K 观察到的类似共沸的负逆行(局部压力最小值)行为,此时 CH4 + CO2 混合气体水合物的平衡压力低于纯 CO2 水合物或纯 CH4 水合物的平衡压力。当 CH4 水合物开采后将 CO2 注入 CH4 剩余储层时,CH4 + CO2 混合气体水合物相中的 CO2 成分可增加到局部压力最低点(如果存在)或沉积物温度高于 Q2 温度时的四相平衡点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


