Solid–Liquid Phase Equilibria of Chrysin in 12 Pure Solvents: Measurement, Model Evaluation, Solvent Effect, and Molecular Simulation
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The solubility of chrysin in 12 pure solvents (ethanol, isobutanol, 1-pentanol, ethyl formate, methyl acetate, butyl acetate, n-propyl acetate, isopropyl acetate, amyl acetate, ethyl propionate, methyl butyrate, and acetonitrile) was determined at different temperatures (T = 290.15 to 323.15 K) and correlated by the Apelblat model, λh equation, non-random two-liquid model, and Wilson model. The results indicated that the solubility of chrysin is positively correlated with temperature, and the Apelblat model presented the most satisfactory regression result by comparing the root mean square deviation and relative average deviation values to those of other models. Furthermore, the related intermolecular simulations and solvation effects were assigned to explore the possible factors determining the solubility of chrysin. The final result confirmed that the dissolution process of chrysin is very complicated, which is not decided by only a single factor.
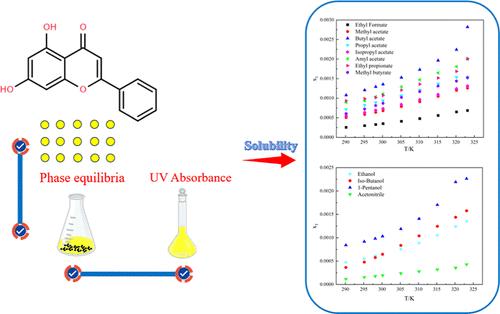
Chrysin 在 12 种纯溶剂中的固液相平衡:测量、模型评估、溶剂效应和分子模拟
测定了不同温度(T = 290.15 至 323.15 K)下菊脂素在 12 种纯溶剂(乙醇、异丁醇、1-戊醇、甲酸乙酯、乙酸甲酯、乙酸丁酯、乙酸正丙酯、乙酸异丙酯、乙酸戊酯、丙酸乙酯、丁酸甲酯和乙腈)中的溶解度,并通过阿佩尔布拉特模型、λh 方程、非随机双液模型和威尔逊模型进行了相关分析。15 至 323.15 K)下的溶解度,并通过阿佩尔布拉特模型、λh 方程、非随机双液模型和威尔逊模型进行了相关分析。结果表明,蛹虫草苷的溶解度与温度呈正相关,通过比较阿佩尔布拉特模型与其他模型的均方根偏差和相对平均偏差值,阿佩尔布拉特模型的回归结果最令人满意。此外,还对相关的分子间模拟和溶解效应进行了分配,以探讨决定菊黄溶解度的可能因素。最终结果证实,蛹虫草苷的溶解过程非常复杂,并非由单一因素决定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


