Studies on Structure, Morphology and Antimicrobial and Anticancer Activities of [BMIM][BF4]-Coated ZnO Nanoparticles
Abstract
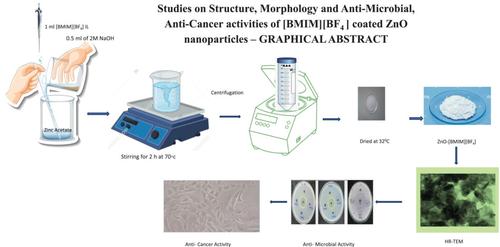
Zinc oxide (ZnO) nanoparticles (NPs) coated with 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid (IL) (ZnO-[BMIM][BF4] NPs) and ZnO NPs without IL coating were both synthesized by co-precipitation. Various analytical methods were used to characterize the synthesized NPs. X-ray diffraction (XRD) analysis revealed that ZnO NPs had a mean crystallite size of 32 nm and that of ZnO-[BMIM][BF4] NPs was 22 nm. IL alters the Fourier transform infrared spectroscopy (FTIR) peaks of the as-prepared ZnO NPs. A blueshift was seen in the absorption peak of ZnO-[BMIM][BF4] NPs as compared to bare ZnO NPs. An examination of the UV–visible (UV–Vis) spectra revealed that the optical bandgap decreases with the addition of IL to ZnO NPs. Scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HR-TEM) investigation revealed the morphology and size of the nanostructured particles. The chemically prepared ZnO-[BMIM][BF4] NPs displayed the irregular spherical-shaped morphologies in the range of nanoscale. Transmission electron microscopy (TEM) showed the conventional hexagonal wurtzite structure of ZnO-[BMIM][BF4] NPs. Synthesized ZnO-NPs in water have a zeta potential of −13.1 mV, showing that they are stable solutions. Thermal behaviour of ZnO NPs was studied using thermogravimetric analysis (TGA) and differential thermal analysis (DTA). The agar-well diffusion technique was used to investigate and compare the antimicrobial activity of synthesized ZnO-[BMIM][BF4] NPs against the following bacteria and fungi: Streptococcus aureus, Pseudomonas aeruginosa and Candida albicans. The MCF7 breast cancer cells were used to assess the anticancer capabilities of ZnO-[BMIM][BF4] NPs at various doses (100 μg/mL, 10 μg/mL, 1 μg/mL, 100 ng/mL, 10 ng/mL and 1 ng/mL) using MTT assays. The range of cell inhibition was determined to be from 19.14% to 70.52%. The percentage of cytotoxicity increases was observed when the concentration of synthesized ZnO-[BMIM][BF4] NPs ranges from 1 ng/mL to 100 μg/mL.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


