Reaction of 2,3,4,5,6-Pentafluorobenzamide with Potassium Hydride: Unexpected Activation of the C–F Bond and Dimerization of Organofluorine Ligand
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The reaction of potassium hydride with 2,3,4,5,6-pentafluorobenzamide (FBAm) in dimethoxyethane results in activation of the C–F bond in the para-position to the C(O)NH2 group followed by dimerization of FBAm to form a potassium salt with one free amide group. The structure of the binuclear reaction product {(DME)2K+[C6F5–C(O)N–C6F4–C(O)NH2]–}2 (I) was determined by X-ray diffraction (CCDC 2311402), the purity of the product was confirmed by NMR spectroscopy.
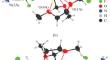
2,3,4,5,6-五氟苯甲酰胺与氢化钾的反应:C-F 键的意外活化和有机氟配体的二聚化
摘要氢化钾与 2,3,4,5,6-五氟苯甲酰胺(FBAm)在二甲氧基乙烷中反应,导致对位的 C-F 键活化为 C(O)NH2 基团,然后 FBAm 二聚形成带有一个游离酰胺基团的钾盐。双核反应产物{(DME)2K+[C6F5-C(O)N-C6F4-C(O)NH2]-}2 (I)的结构由 X 射线衍射法(CCDC 2311402)确定,产物的纯度由核磁共振光谱法确认。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


