Correlation of Urinary Soluble CD163 Levels With Disease Activity and Treatment Response in IgA Nephropathy
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Introduction
The TESTING trial demonstrated that corticosteroids reduce the risk of kidney failure in patients with IgA nephropathy (IgAN) but increase the risk of serious adverse events. Reliable noninvasive biomarkers are needed to identify patients who would benefit most from corticosteroid therapy. Previous studies suggest glomerular macrophage infiltration is associated with response to immunosuppressive therapy in IgAN and urinary soluble CD163 ([u-sCD163], a marker of alternatively activated macrophages [M2]c macrophage) is correlated with clinical remission in vasculitis. This study aims to investigate the association between u-sCD163 and response of steroids therapy in IgAN.
Methods
We measured u-sCD163 in patients from a large IgAN cohort and Chinese participants of the TESTING trial. The correlation of baseline or serial u-sCD163 and their response of corticosteroids therapy or kidney outcomes were investigated.
Results
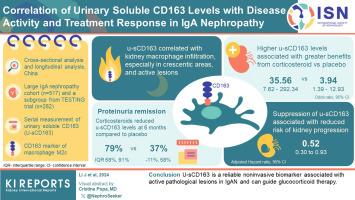
In cross-sectional analysis, u-sCD163 levels correlated with kidney macrophage infiltration, especially in crescentic areas, and with active lesions. Subgroup analysis of the TESTING cohort showed higher levels u-sCD163 were associated with greater benefits from corticosteroids therapy in proteinuria remission (odds ratio, 35.56 [95% confidence interval, CI: 7.62–292.34] vs. 3.94 [95% CI: 1.39–12.93], P for interaction: 0.036). Corticosteroids therapy significantly reduced u-sCD163 levels at 6 months compared to placebo group (79% [interquartile range: 58%–91%] vs. 37% [−11% to 58%], P <0.001). There was no difference in the suppressive effects on u-sCD163 by either dosage of corticosteroids (full and reduced-dose). The suppression of u-sCD163 was significantly associated with a reduced risk of kidney progression events (adjusted hazard ratio: 0.52, 95% CI: 0.30–0.93, P = 0.027).
Conclusion
u-sCD163 is a reliable noninvasive biomarker associated with active pathological lesions in IgAN and can guide glucocorticoid therapy.
尿液可溶性 CD163 水平与 IgA 肾病的疾病活动性和治疗反应的相关性
TESTING试验表明,皮质类固醇可降低IgA肾病(IgAN)患者发生肾衰竭的风险,但会增加发生严重不良事件的风险。需要可靠的非侵入性生物标志物来确定哪些患者最受益于皮质类固醇治疗。先前的研究表明,肾小球巨噬细胞浸润与IgAN患者对免疫抑制疗法的反应有关,尿液可溶性CD163([u-sCD163],一种替代活化巨噬细胞[M2]c巨噬细胞的标记物)与脉管炎的临床缓解有关。本研究旨在探讨u-sCD163与IgAN患者类固醇治疗反应之间的关系。我们测量了来自大型 IgAN 队列的患者和 TESTING 试验的中国参与者的 u-sCD163。我们研究了基线或序列u-sCD163与皮质类固醇治疗反应或肾脏预后的相关性。在横断面分析中,u-sCD163水平与肾脏巨噬细胞浸润(尤其是在新月体区域)和活动性病变相关。对 TESTING 队列进行的亚组分析显示,u-sCD163 水平越高,蛋白尿缓解时从皮质类固醇治疗中获益越多(比值比 35.56 [95% 置信区间:7.62-292.34] vs. 3.94 [95% 置信区间:1.39-12.93],交互作用为 0.036):0.036).与安慰剂组相比,皮质类固醇治疗可显著降低6个月时的u-sCD163水平(79% [四分位间范围:58%-91%] vs. 37% [-11% to 58%],<0.001)。两种剂量的皮质类固醇(全量和减量)对u-sCD163的抑制作用没有差异。u-sCD163的抑制与肾脏恶化风险的降低显著相关(调整后危险比:0.52,95% CI:0.30-0.93,=0.027)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


