Coordination Ability of Phosphavinyl(oxo and thioxo)phosphoranes (P═C–P═X; X = O, S) toward Transition Metals
IF 2.9
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The coordination preferences of phosphavinylphosphorane species with the Mes*P═C(Cl)–PR1,R2(═X) general formula (Mes* = 2,4,6-trit-butylphenyl; X = O, S; R1 = Cl, R2 = 2,4,6-trii-propylphenyl; R1 = R2 = i-propyl) were evaluated in the presence of Au, W and Pd transition metals, by means of both experimental and theoretical investigations. Targeted compounds were characterized in solution by multinuclear NMR spectroscopy and HRMS, while for several cases, the solid-state structures were measured through X-ray diffraction. The preference for the coordination through the sulfur lone pair toward gold fragment was observed in the solid state, yet computational data highlight a possible fluxional character occurring in solution. Density functional theory mechanistic explorations suggest that the AuCl moiety shuttles between the P(III) and S atoms. Tungsten phosphavinylphosphorane complexes are stabilized by the phosphavinylphosphorane ligands in a bidentate coordination mode, involving both the P(III) and chalcogen atom. The coordination compounds with palladium fragments lead to the corresponding chelate compounds and the subsequent activation of a C–H bond from an ortho-methyl group of the Mes* group was observed if the P═C–P(═X) unit contained a Cl or O atom on the P(V) atom.
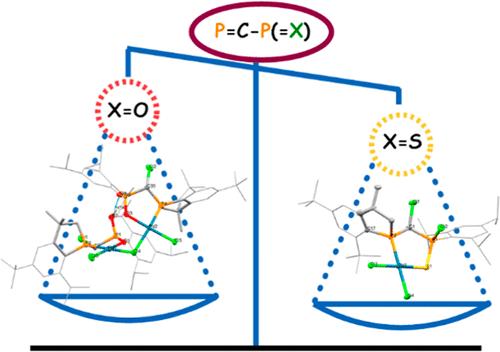
磷酰基(氧代和硫代)磷烷(P═C-P═X;X = O、S)与过渡金属的配位能力
通过实验和理论研究,评估了通式为 Mes*P═C(Cl)-PR1,R2(═X)(Mes* = 2,4,6-三丁基苯基;X = O,S;R1 = Cl,R2 = 2,4,6-三丙基苯基;R1 = R2 = i-丙基)的磷酰基磷烷物种在金、钨和钯过渡金属存在下的配位偏好。通过多核核磁共振光谱和 HRMS 对溶液中的目标化合物进行了表征,同时通过 X 射线衍射对几种化合物的固态结构进行了测量。在固态中观察到,硫孤对对金片段有配位偏好,但计算数据强调了溶液中可能出现的通性特征。密度泛函理论机理探索表明,AuCl 分子在 P(III) 原子和 S 原子间穿梭。膦酰基膦烷钨配合物通过膦酰基膦烷配体以双齿配位模式稳定下来,其中涉及 P(III)原子和钙原子。如果 P═C-P(═X) 单元的 P(V)原子上含有一个 Cl 原子或 O 原子,则可观察到钯片段的配位化合物会导致相应的螯合物,并随后从 Mes* 基团的一个正交甲基活化出一个 C-H 键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


