Flash Communication: A Ferrous Adduct of a Phosphanylidene-σ4-phosphorane
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
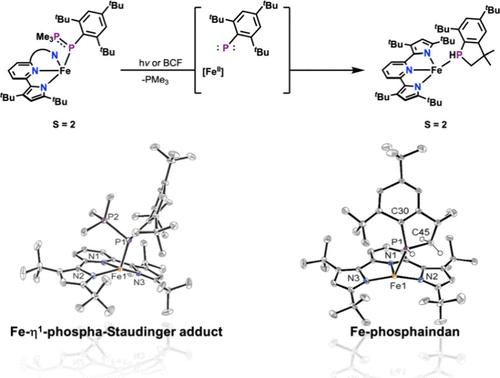
The sterically encumbered phosphanylidene-σ4-phosphorane Mes*PPMe3 (Mes* = 2,4,6-tBu3C6H2) smoothly displaces Et2O in [(tBupyrr2py)Fe(OEt2)] (1-OEt2) (tBupyrr2py2– = 3,5-tBu2-bis(pyrrolyl)pyridine) to form a rare example of a η1-phospha-Staudinger adduct of Fe, namely, [(tBupyrr2py)Fe(Mes*PPMe3)] (1-Mes*PPMe3) in 64% yield. Complex 1-Mes*PPMe3 is a ferrous, S = 2 system and quite thermally stable, but in the presence of B(C6F5)3 and photolysis, it forms the phosphaindan ferrous adduct [(tBupyrr2py)Fe(phosphaindan)] (1-phosphaindan) (phosphaindan = HPCH2C(Me2)-2,4-tBu2C6H2) along with Me3PB(C6F5)3. In the absence of Lewis acid, photolysis of 1-Mes*PPMe3 results instead in the formation of free phosphaindan and the PMe3 ferrous adduct [(tBupyrr2py)Fe(PMe3)] (1-PMe3) thus suggesting that dissociation of the phosphanylidene-σ4-phosphorane precedes the formation of a transient phosphinidene fragment Mes*P. All phosphorus adducts of 1 were crystallographically characterized and show quite similar Fe–P distances (2.4685(5) for 1-Mes*PPMe3; 2.5062(7) for 1-phosphaindan; and 2.4323(8) Å for 1-PMe3).
闪电通讯:亚磷酰σ4-磷烷的亚铁加合物
立体包被的亚膦酰-σ4-磷烷 Mes*PPMe3 (Mes* = 2,4,6-tBu3C6H2)顺利取代了 [(tBupyrr2py)Fe(OEt2)](1-OEt2)(tBupyrr2py2- = 3、5-tBu2-双(吡咯基)吡啶)中形成一种罕见的 η1-phospha-Staudinger 加合物,即[(tBupyrr2py)Fe(Mes*PPMe3)](1-Mes*PPMe3),收率为 64%。络合物 1-Mes*PPMe3 是一个亚铁、S = 2 体系,热稳定性相当高,但在 B(C6F5)3 和光解作用下,它会与 Me3PB(C6F5)3 一起形成磷丹亚铁加合物[(tBupyrr2py)Fe(phosphaindan)](1-phosphaindan)(磷丹 = HPCH2C(Me2)-2,4-tBu2C6H2)。在没有路易斯酸的情况下,1-Mes*PPMe3 的光解反而会形成游离的磷丹和 PMe3 亚铁加合物[(tBupyrr2py)Fe(PMe3)](1-PMe3),这表明在形成瞬时亚膦片段 Mes*P 之前,亚膦-σ4-磷烷已经解离。1 的所有磷加合物都具有晶体学特征,并显示出非常相似的铁-磷距离(1-Mes*PPMe3 为 2.4685(5);1-phosphaindan 为 2.5062(7);1-PMe3 为 2.4323(8) Å)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


