Rhodium-Catalyzed Arene Alkenylation: Selectivity and Reaction Mechanism as a Function of In Situ Oxidant Identity
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
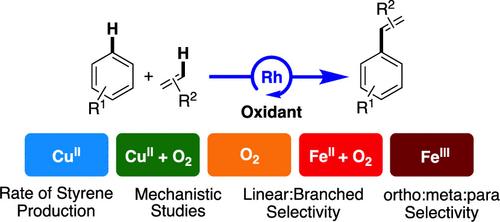
Rhodium catalyzed arene alkenylation reactions with arenes and olefins using dioxygen as the direct oxidant (e.g., ACS Catal. 2020, 10, 11519), Cu(II) carboxylates (e.g., Science 2015, 348, 421; J. Am. Chem. Soc. 2017, 139, 5474) or Fe(III) carboxylate clusters (e.g., ACS Catal. 2024, 14, 10295), in the presence or absence of dioxygen, have been reported. These processes involve heating catalyst precursor [(η2-C2H4)2Rh(μ-OAc)]2, olefin, arene, and oxidant at temperatures between 120 and 200 °C. Herein, we report comparative studies of Rh-catalyzed arene alkenylation as a function of oxidant identity. This work includes comparisons of catalysis using Cu(II) carboxylates in the presence and absence of dioxygen, catalysis with only dioxygen as the oxidant, and Fe(III) carboxylates in the presence and absence of dioxygen. We report studies of catalysis with each oxidant including reagent concentration dependencies and kinetic isotope effect experiments using C6H6 or C6D6 and protio- or deutero carboxylic acid. Additionally, we probe ortho/meta/para regioselectivity for reactions of ethylene with monosubstituted arenes and Markovnikov/anti-Markovnikov selectivity with monosubstituted olefins. These studies indicate that the variation of oxidant identity impacts catalyst speciation, the reaction mechanism, and the reaction rate. Consequently, distinct Markovnikov/anti-Markovnikov and ortho/meta/para selectivities are observed for catalysis with each oxidant.
铑催化的烯炔化反应:选择性和反应机理与原位氧化剂特性的关系
有报道称,在有或没有二氧的情况下,使用二氧作为直接氧化剂(例如,ACS Catal.这些工艺涉及在 120 至 200 °C 的温度下加热催化剂前体 [(η2-C2H4)2Rh(μ-OAc)]2 、烯烃、炔和氧化剂。在此,我们报告了 Rh 催化的炔烯化反应与氧化剂特性的比较研究。这项工作包括比较在有二氧和无二氧条件下使用 Cu(II) 羧酸盐的催化作用、仅使用二氧作为氧化剂的催化作用以及在有二氧和无二氧条件下使用 Fe(III) 羧酸盐的催化作用。我们报告了对每种氧化剂催化作用的研究,包括试剂浓度依赖性以及使用 C6H6 或 C6D6 和原羧酸或脱羧酸进行的动力学同位素效应实验。此外,我们还探究了乙烯与单取代烯烃反应的正/正/副区域选择性,以及与单取代烯烃反应的马可夫尼科夫/反马可夫尼科夫选择性。这些研究表明,氧化剂特性的变化会影响催化剂规格、反应机理和反应速率。因此,在使用每种氧化剂进行催化时,都会观察到不同的马可夫尼科夫/反马可夫尼科夫和正/元/副选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


