Facile photopatterning of perfusable microchannels in hydrogels for microphysiological systems
IF 13.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
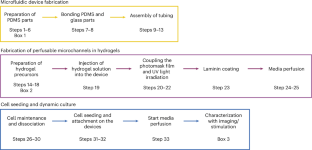
Perfusable hydrogels have garnered substantial attention in recent years for the fabrication of microphysiological systems. However, current methodologies to fabricate microchannels in hydrogel platforms involve sophisticated equipment and techniques, which hinder progress of the field. In this protocol, we present a cost-effective, simple, versatile and ultrafast method to create perfusable microchannels of complex shapes in photopolymerizable hydrogels. Our method uses one-step UV photocross-linking and a photomask printed on inexpensive transparent films, to photopattern both synthetic (PEG-norbornene) and natural (hyaluronic acid-norbornene) hydrogels in just 0.8 s. Moreover, these perfusable hydrogels are fully integrated into a custom-made microfluidic device that allows continuous fluid perfusion when connected to an external pump system. This methodology can be easily reproduced by professionals with basic laboratory skills and a fundamental knowledge of polymers and materials science. In this protocol, we demonstrate the functionality of our photopatterned hydrogels by seeding human endothelial cells into the microchannels, culturing them under dynamic conditions for 7 d, and exposing them to inflammatory stimuli to elicit cellular responses. This highlights the versatility of our platform in fabricating microphysiological systems and different microenvironments. The fabrication of perfusable channels within the hydrogels, including the fabrication of the microfluidic devices, requires ~3 d. The development of the cell-seeded microphysiological system, including the stimulation of cells, takes ~7 d. In conclusion, our approach provides a straightforward and widely applicable solution to simplify and reduce the cost of biofabrication techniques for developing functional in vitro models using perfusable three-dimensional hydrogels. A cost-effective, facile, versatile and ultrafast methodology to fabricate perfusable microchannels of complex shapes in photopolymerizable hydrogels without the need for specialized equipment or sophisticated protocols.

水凝胶中可灌注微通道的简易光图案化,用于微生理系统
近年来,可灌注水凝胶在微物理系统的制造方面引起了广泛关注。然而,目前在水凝胶平台中制造微通道的方法涉及复杂的设备和技术,阻碍了该领域的发展。在本方案中,我们提出了一种经济、简单、多功能和超快的方法,用于在可光聚合水凝胶中制造形状复杂的可灌注微通道。我们的方法使用一步紫外光交联技术和印在廉价透明薄膜上的光掩模,在短短 0.8 秒内对合成(PEG-降冰片烯)和天然(透明质酸-降冰片烯)水凝胶进行光图案化。只要具备基本的实验室技能以及聚合物和材料科学的基础知识,专业人员就能轻松复制这种方法。在本实验中,我们将人类内皮细胞播种到微通道中,在动态条件下培养 7 天,并将其暴露在炎症刺激下以激发细胞反应,从而展示了光图案水凝胶的功能。这凸显了我们的平台在制造微物理系统和不同微环境方面的多功能性。总之,我们的方法提供了一种直接而广泛适用的解决方案,可简化生物制造技术并降低其成本,从而利用可灌注三维水凝胶开发功能性体外模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


