Neoadjuvant gemcitabine–cisplatin plus tislelizumab in persons with resectable muscle-invasive bladder cancer: a multicenter, single-arm, phase 2 trial
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
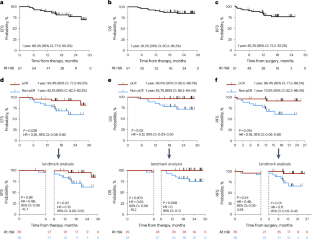
Programmed death 1 blockade (tislelizumab) has been approved for metastatic urothelial carcinoma but not as part of neoadjuvant therapy for muscle-invasive bladder cancer (MIBC). In this multicenter single-arm trial (ChiCTR2000037670), 65 participants with cT2-4aN0M0 MIBC received neoadjuvant gemcitabine–cisplatin plus tislelizumab; 57 of them underwent radical cystectomy (RC). The primary endpoint of pathologic complete response (pCR) rate was 50.9% (29/57, 95% confidence interval (CI) 37.3–64.4%) and the pathologic downstaging (secondary endpoint) rate was 75.4% (43/57, 95% CI 62.2–85.9%) in participants undergoing RC. Genomic and transcriptomic analyses revealed three MIBC molecular subtypes (S): S1 (immune-desert) with activated cell-cycle pathway, S2 (immune-excluded) with activated transforming growth factor-β pathway and S3 (immune-inflamed) with upregulated interferon-α and interferon-γ response. Post hoc analysis showed pCR rates of 16% (3/19, S1), 77% (10/13, S2) and 80% (12/15, S3) (P = 0.006). In conclusion, neoadjuvant gemcitabine–cisplatin plus tislelizumab for MIBC was compatible with an enhanced pCR rate. Li et al. perform a phase 2 single-arm clinical trial of neoadjuvant chemotherapy plus checkpoint blockade in participants with resectable muscle-invasive bladder cancer and conduct genomic and transcriptomic profiling to describe molecular subtypes.

对可切除的肌肉浸润性膀胱癌患者进行吉西他滨-顺铂加替斯利珠单抗的新辅助治疗:一项多中心、单臂、2 期试验
程序性死亡 1(tislelizumab)阻断疗法已被批准用于治疗转移性尿路上皮癌,但未被批准作为肌层浸润性膀胱癌(MIBC)新辅助疗法的一部分。在这项多中心单臂试验(ChiCTR2000037670)中,65名患有cT2-4aN0M0 MIBC的患者接受了吉西他滨-顺铂加替斯利珠单抗的新辅助治疗,其中57人接受了根治性膀胱切除术(RC)。接受根治性膀胱切除术的患者的主要终点病理完全应答率(pCR)为50.9%(29/57,95%置信区间(CI)为37.3-64.4%),病理分期(次要终点)为75.4%(43/57,95%置信区间(CI)为62.2-85.9%)。基因组和转录组分析显示,MIBC 有三种分子亚型(S):S1(免疫凋亡)具有激活的细胞周期通路,S2(免疫排斥)具有激活的转化生长因子-β通路,S3(免疫炎症)具有上调的干扰素-α和干扰素-γ反应。事后分析显示,pCR 率分别为 16%(3/19,S1)、77%(10/13,S2)和 80%(12/15,S3)(P = 0.006)。总之,新辅助吉西他滨-顺铂加替斯利珠单抗治疗MIBC可提高pCR率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


