Electrochemical mechanistic study of chlorpromazine oxidation in the presence of l-cysteine by digital simulation program: Introducing of EEC and EC'EC mechanisms
Abstract
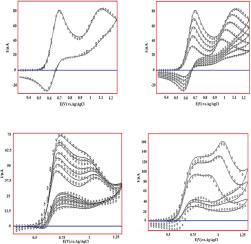
New electrochemical mechanisms of chlorpromazine (CPZ) as one of the derivatives of phenothiazine and chlorpromazine in the presence of l-cysteine as an amino acid at 0.3 - 1.3 V on boron-doped diamond (BDD) electrode surface were studied using cyclic voltammetry (CV) technique for the first time. The results indicated that the electrochemical oxidation mechanism of chlorpromazine is EEC. Furthermore, the results of studying the electrochemical behavior of chlorpromazine in the presence of l-cysteine shows an electrocatalytic mechanism. According to these results, this system is implicated in the EC'EC mechanism. For both mechanisms, we studied the impact of increasing the concentration and rate of potential scanning. Digital simulation (Digital Simulation3 program) analyses were conducted using these mechanisms for cyclic voltammograms obtained on the boron-doped diamond electrode surface. Kinetic data were extracted from these voltammograms through digital simulations to estimate the heterogeneous rate constant (ks), equilibrium constant (Keq), charge-transfer coefficient (α), chemical rate constant (kc) and diffusion coefficient (D) by comparing the experimental responses with the simulated results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


